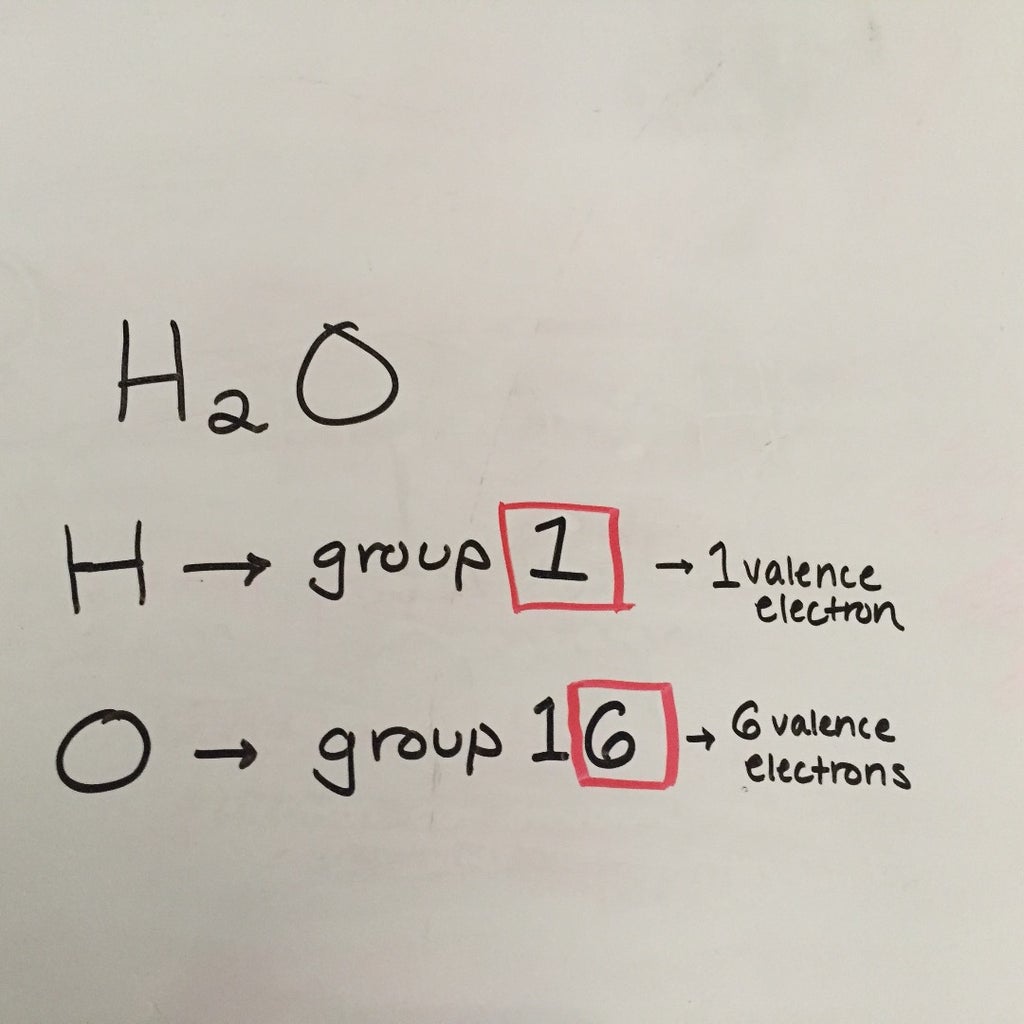To Draw A Lewis Structure One Must Know The
To Draw A Lewis Structure One Must Know The - Write the formula of each compound using the chemical symbols of each element: Place least electronegative element in center and draw single bonds from the central atom to other atoms. In this animated lecture, i will teach you drawing lewis structure of different compounds. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. There is no difference between the length of the two bonds in so 2,.
The sum of the valence electrons is. General rules for drawing lewis structures. Determine how many electrons must be added to the. To learn more about how to draw lewis structure. Web draw lewis structures for molecules. Web after drawing a lewis structure, one should. Web ask me questions on facebook:
How to Draw a Lewis Structure
Determine how many electrons must be added to the. Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. Lewis symbols we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. To find the number of valence electrons, you will have to do a simple computation..
How to Draw Lewis Structures YouTube
Web to begin, you must know two essential rules for drawing lewis structures. To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules: How many double bonds are in the lewis structure for hydrogen fluoride, hf? Web electron dot structures or lewis dot formula can be drawn if.
How To Draw Lewis Structures Abilitystop
A lewis structure also helps to make a prediction about the geometry of a molecule. Lewis structures can also be useful in predicting. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Web after drawing a lewis structure, one should..
Lesson 7 Drawing Lewis Structures YouTube
Determine the total number of valence (outer shell) electrons. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Web a formal charge does not represent a true charge on an atom in a covalent bond but is simply used to.
How to Draw Lewis Structures 5 Steps Instructables
Write the formula of each compound using the chemical symbols of each element: Web in the lewis structures listed below, m and x represent various elements in the third period of the periodic table. To find the number of valence electrons, you will have to do a simple computation. Web ask me questions on facebook:.
Beginner's Guide to Drawing Lewis Structures YouTube
To find the number of valence electrons, you will have to do a simple computation. Count the total number of valence electrons the first step is determining the count of valence electrons found in all the atoms that make the molecule you are dealing with. Web this organic chemistry video tutorial explains how to draw.
How to Draw a Lewis Structure
Interestingly enough, neither of these structures is correct. Determine how many electrons must be added to the. Web in the lewis structures listed below, m and x represent various elements in the third period of the periodic table. Symbol for an element or monatomic ion that uses a dot to represent each valence electron in.
Lewis Dot Structure Definition, Examples, and Drawing
Lewis structures can also be useful in predicting. Move them around until all the atoms have 8 in their outer orbits. There is no difference between the length of the two bonds in so 2,. Place least electronegative element in center and draw single bonds from the central atom to other atoms. To find the.
OneClass Draw complete Lewis structure, including lone pairs, for the
Shared pairs of electrons are drawn as lines between atoms, while. None how many electrons must be shown in the lewis structure of the hydroxide ion? Move them around until all the atoms have 8 in their outer orbits. · 1 · jul 3 2018 questions Web this organic chemistry video tutorial explains how to.
How to Draw Lewis Structures RodneytaroBall
The video covers the basic lewis structures you'll see in an introductory chemistry class. Two (a pair of) valence electrons that are not used to form a covalent bond. Determine the total number of valence (outer shell) electrons. Shared pairs of electrons are drawn as lines between atoms, while. To find the number of valence.
To Draw A Lewis Structure One Must Know The Web as a result, they must be equally satisfactory representations of the molecule. Web () you can use a drawing or use small beads or objects to represent electrons and work out the electron arrangement. Two (a pair of) valence electrons that are not used to form a covalent bond. Determine the total number of valence (outer shell) electrons. Web updated on january 29, 2020 a lewis structure is a graphic representation of the electron distribution around atoms.
Web Draw Lewis Structures For Molecules.
Web this lecture is about how to draw lewis structure easily. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Web in this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely lewis symbols and lewis structures. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.
General Rules For Drawing Lewis Structures.
Shared pairs of electrons are drawn as lines between atoms, while. Web to begin, you must know two essential rules for drawing lewis structures. Write the formula of each compound using the chemical symbols of each element: Water (h 2 o), dinitrogen monoxide (nitrous oxide, n 2 o), acetic acid (c 2 h 4 o 2 ).
Symbol For An Element Or Monatomic Ion That Uses A Dot To Represent Each Valence Electron In The Element Or Ion.
To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules: Count the total number of valence electrons the first step is determining the count of valence electrons found in all the atoms that make the molecule you are dealing with. Web ask me questions on facebook: We will also go over what the octet rule is and all the exceptions that go along with this.
Lewis Structures Can Also Be Useful In Predicting.
Web this organic chemistry video tutorial explains how to draw lewis structures using a simple method. A lewis structure also helps to make a prediction about the geometry of a molecule. It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. Since valence electrons are typically represented as dots.