P Orbital Drawing
P Orbital Drawing - In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and 67% p character. Four of them fill the 1s and 2s orbitals. 22 similar questions q 1 (a) draw the electron dot structure for: How likely it is to form bonds, and with which other elements. As the value of l increases, the number of orbitals in a given subshell increases, and the shapes of the orbitals become more complex.
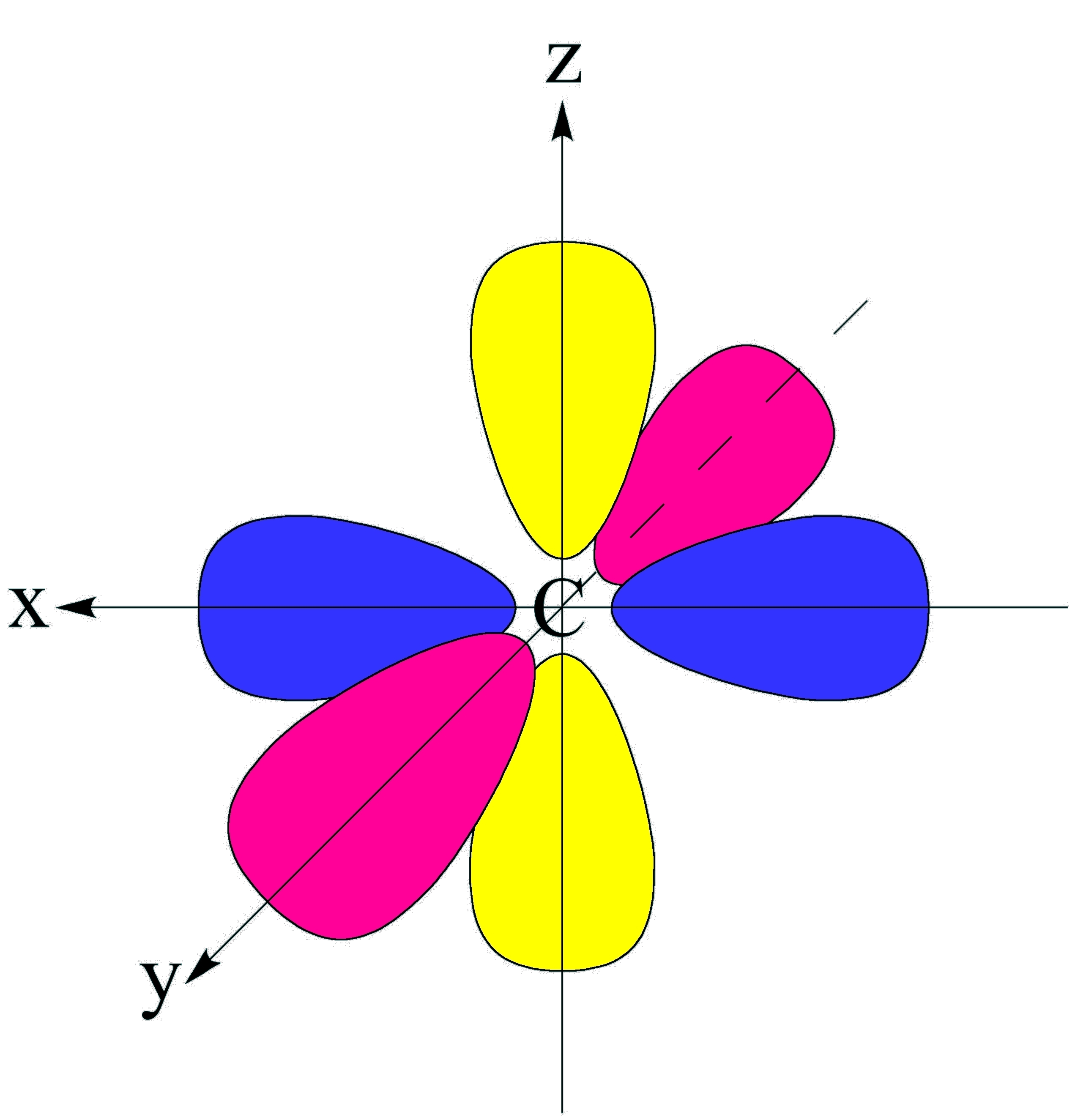
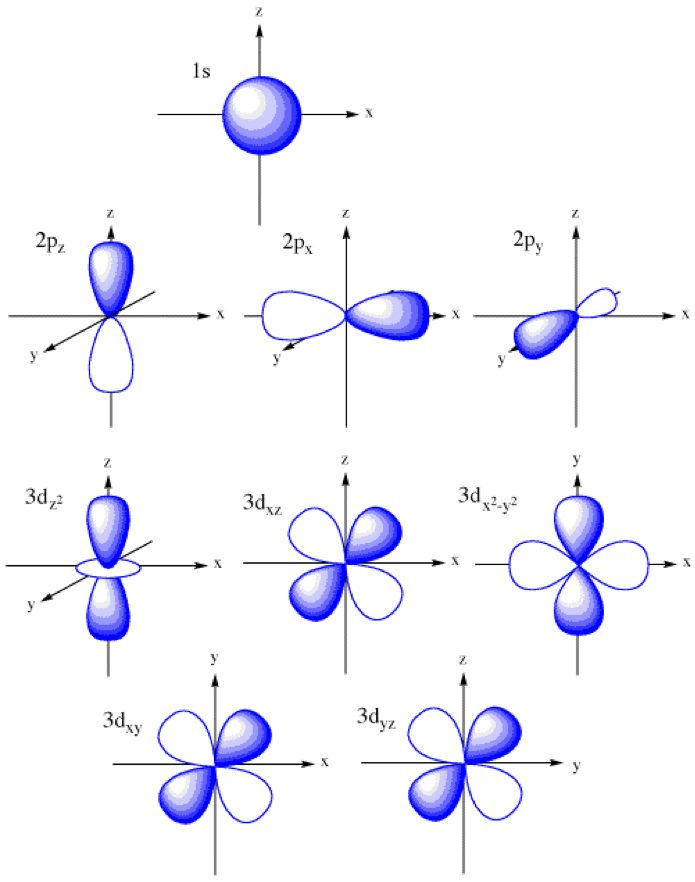
F 2 view solution q 2 Imagine shells around the nucleus, that get bigger and bigger. How likely it is to form bonds, and with which other elements. So a p orbital is just that dumbbell shape. Four of them fill the 1s and 2s orbitals. A p orbital consists of two lobes of electron density on either side of the nucleus. It only has s orbitals.
[Solved] sketch sigma and pi bond from p orbital Course Hero
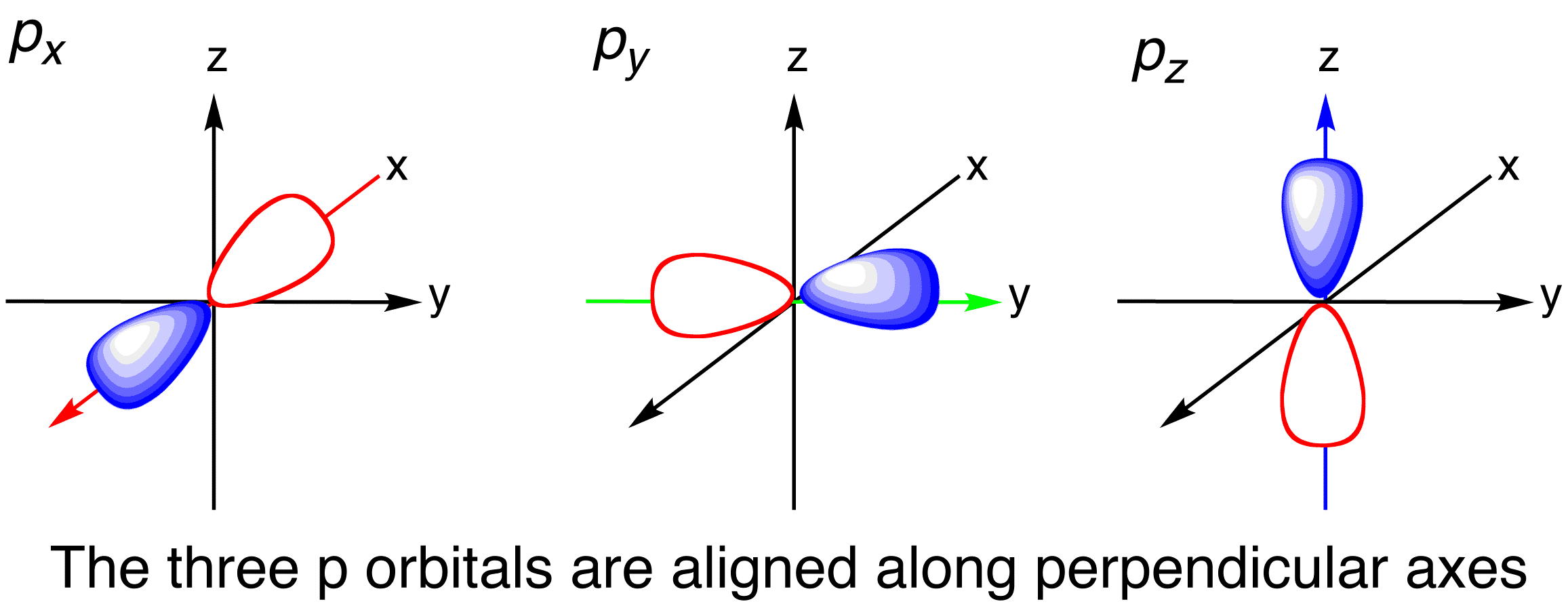
An s orbital is a sphere. Web a p orbital is shaped like 2 identical balloons tied together at the nucleus. Imagine a horizontal plane through the nucleus, with one lobe of the orbital above the plane and the other beneath it; Draw the x, y, z axes first and then draw your orbital on.
Illustrated Glossary of Organic Chemistry Orbital
Web p orbitals (l=1) only s orbitals are spherically symmetrical. Web as we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for predicting the reactivity of an element: Web this means that the s orbital can contain up to two.
Atomic orbitals explained polizhuge
Those electrons can participate in resonance. 2) always shade your orbitals appropriately to represent the signs of the wave function. Web 1) draw each orbital superimposed on a labeled coordinate system (i.e. There is a zero probability of finding the electron on that plane. Web there are three degenerate 2p orbitals (m l = −1,.
2. What is the shape of p orbital? Brainly.ph
The three p orbitals are at right angles to each other and have a lobed shape. Web a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Web p.
Shape of porbitals in 3D
Four of them fill the 1s and 2s orbitals. Web there are three degenerate 2p orbitals (m l = −1, 0, +1) and the electron can occupy any one of these p orbitals. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. The.
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry
This means that you can only put two electrons (with opposite spin) in the first shell. Web the shapes of p, d and f orbitals are described verbally here and shown graphically in the orbitals table below. Four of them fill the 1s and 2s orbitals. Carbon (atomic number 6) has six electrons. Web there.
Describe the shapes of s and p orbitals.
Web draw an mo cartoon of a sigma bonding orbital formed by the overlap of two p orbitals between two oxygen atoms. Orbitals with total angular momentum quantum number l = 1 are called p orbitals. For a p orbital, draw a figure eight; Web p orbitals (l=1) only s orbitals are spherically symmetrical. The.
How To Draw Orbitals Deepcontrol3
The orbital shows where there is a 95% chance of finding a particular electron. Web the shapes of p, d and f orbitals are described verbally here and shown graphically in the orbitals table below. Four of them fill the 1s and 2s orbitals. A fundamental principle of these theories is that as atoms bond.
Shapes of Atomic Orbitals — Overview & Examples Expii
This means that you can only put two electrons (with opposite spin) in the first shell. Web a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. There will.
quantum chemistry How do 1s and 2p orbitals overlap? Chemistry
In two dimensions, we draw it as a circle. Remember that l must always be less than n. This means that you can only put two electrons (with opposite spin) in the first shell. Web for an s orbital, draw a circle; The p sub shell can hold a maximum of six electrons as there.
P Orbital Drawing Carbon (atomic number 6) has six electrons. So a p orbital is just that dumbbell shape. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals. There is a zero probability of finding the electron on that plane. For a p orbital, draw a figure eight;
As The Value Of L Increases, The Number Of Orbitals In A Given Subshell Increases, And The Shapes Of The Orbitals Become More Complex.
Web draw an mo cartoon of a sigma bonding orbital formed by the overlap of two p orbitals between two oxygen atoms. H 2s (c) draw the electron dot structure for: The p sub shell can hold a maximum of six electrons as there are three orbitals within this sub shell. Web for an s orbital, draw a circle;
Web P Orbitals (L=1) Only S Orbitals Are Spherically Symmetrical.
It only has s orbitals. Label the positions of the oxygen nuclei with the symbol o. Web a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. An s orbital is a sphere.
Web As We Will See Below, The Periodic Table Organizes Elements In A Way That Reflects Their Number And Pattern Of Electrons, Which Makes It Useful For Predicting The Reactivity Of An Element:
We classified the different orbital into shells and sub shells to distinguish them more easily. This type of hybridization is required whenever an atom is surrounded by three groups of electrons. The orbital diagram for phosphorus is drawn with 5 orbitals. Web what is the orbital diagram for phosphorus (p)?
Web There Are Three Degenerate 2P Orbitals (M L = −1, 0, +1) And The Electron Can Occupy Any One Of These P Orbitals.
The phosphorus orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the remaining. Imagine a horizontal plane through the nucleus, with one lobe of the orbital above the plane and the other beneath it; The orbital shows where there is a 95% chance of finding a particular electron. Carbon (atomic number 6) has six electrons.