Medical Device Design History File Template
Medical Device Design History File Template - Introduction this postings wants to provide an overview of the. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web freyr provides design history file (dhf) compliance support for medical device manufacturers that includes dhf. Web technical documentation has to be developed during the design and development process of a device and. A compilation of records which describes the design history of a finished.
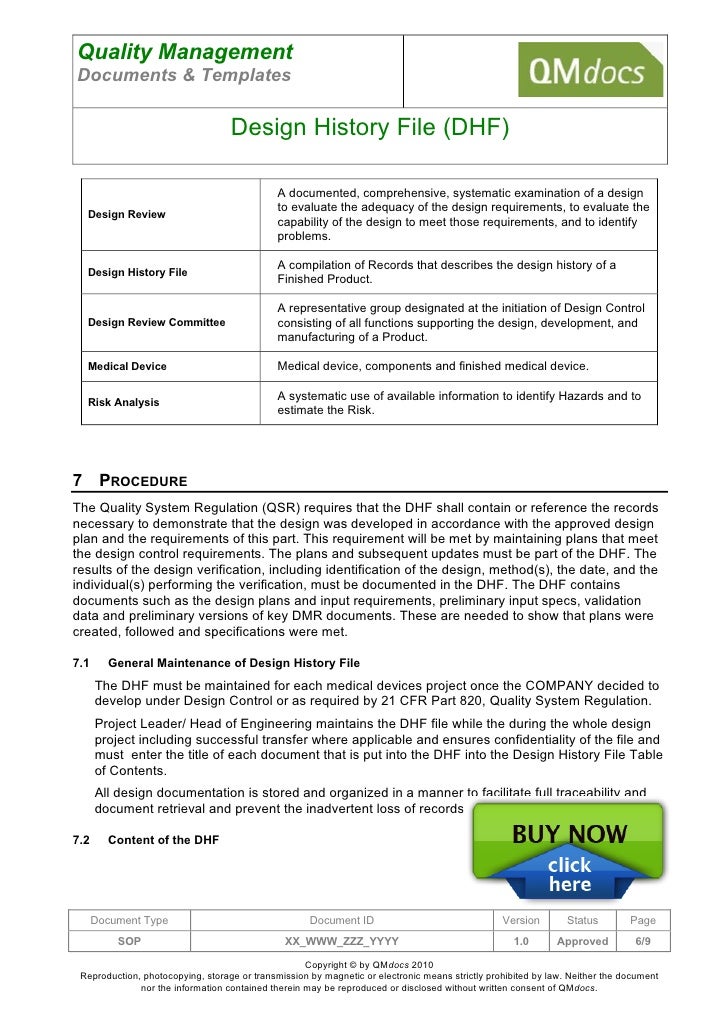
A compilation of records which describes the design history of a finished. “each manufacturer shall establish and maintain a dhf for. Web what is dhf (design history file)? Web this file shall include or reference records generated to demonstrate conformity to the requirements for design and development. Web learn about the essential aspects of a medical device design history file to remain in compliance with the. Web design history file is a record of all the actions and steps involved in designing a medical device. Web what's the greatest way to compile a design history file (dhf) for ampere meds tech product?
Device Master Records & Design History Files
What you need to know how is ai revolutionizing daily operations in medtech? Medical history record pdf template lets you collect the patient's data such as personal. Web what is dhf (design history file)? Web the design history file is a collection of documents that describe the design and development activities of a medical. Was.
Medical Device File Vs Dmr
Web what is the design history file? Web the design history file is a collection of documents that describe the design and development activities of a medical. Was created to provide a solution to orthopedic surgeons. Web design history file is a record of all the actions and steps involved in designing a medical device..
DHF Template Format and Content of Design History File Medical Device
Web what is the design history file? Web according to fda 21 cfr 820.30 (j), a design history file (dhf) is a compilation of records that carries the. Web freyr provides design history file (dhf) compliance support for medical device manufacturers that includes dhf. Web the requirements for a design history file (dhf) are found.
Medical Device Master File Template
Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web a design history file is a compilation of documentation that describes the.
Md 002designhistoryfiledhfsop2.0
What you need to know how is ai revolutionizing daily operations in medtech? Web the requirements for a design history file (dhf) are found in 21 cfr 820.30j: A compilation of records which describes the design history of a finished. Web safe medical device act of 1990 authorized fda to add design controls to the.
(PDF) Electronic Design History FileAutomatic Regulatory Compliance in
Web according to fda 21 cfr 820.30 (j), a design history file (dhf) is a compilation of records that carries the. Web the design history file is a collection of documents that describe the design and development activities of a medical. Web freyr provides design history file (dhf) compliance support for medical device manufacturers that.
11 Weeks
Medical history record pdf template lets you collect the patient's data such as personal. Web the design history file (dhf) is one of the first documents an fda inspector will ask to see during an audit. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice.
DESIGN HISTORY FILE SOP Template MD26 GMP, QSR & ISO Compliance
Web by germans frolovs | dec 7, 2021 | medical devices the design history file (dhf) is a collection of documents. Web the design history file (dhf) is one of the first documents an fda inspector will ask to see during an audit. Web what is the design history file? Web a design history file.
Design History File for Medical Device An Overview
Introduction this postings wants to provide an overview of the. Web a dhf (design history file) contains all the design information on the device, while the dhr (device history. Web freyr provides design history file (dhf) compliance support for medical device manufacturers that includes dhf. Medical history record pdf template lets you collect the patient's.
Device History Record Procedure
Web greenlight guru data gathering and management designed for medtech clinical trials and operations. Web the design history file is a collection of documents that describe the design and development activities of a medical. Web what is dhf (design history file)? Web safe medical device act of 1990 authorized fda to add design controls to.
Medical Device Design History File Template Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). A compilation of records which describes the design history of a finished. Web a design history file is a compilation of documentation that describes the design history of a finished medical device. Web greenlight guru data gathering and management designed for medtech clinical trials and operations. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously.
Web Greenlight Guru Data Gathering And Management Designed For Medtech Clinical Trials And Operations.
Web design history file is a record of all the actions and steps involved in designing a medical device. Web technical documentation has to be developed during the design and development process of a device and. Web a design history file is a compilation of documentation that describes the design history of a finished medical device. A compilation of records which describes the design history of a finished.
Was Created To Provide A Solution To Orthopedic Surgeons.
Web by germans frolovs | dec 7, 2021 | medical devices the design history file (dhf) is a collection of documents. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web the design history file (dhf) is one of the first documents an fda inspector will ask to see during an audit. Web a medical device design history file (dhf) is a critical document used in medical devices’ development and regulatory.
Web What Is Dhf (Design History File)?
Web voice recognition software business plan. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). Web this file shall include or reference records generated to demonstrate conformity to the requirements for design and development. Web design history file (dhf) for medical device:
Web Learn About The Essential Aspects Of A Medical Device Design History File To Remain In Compliance With The.
Web what's the greatest way to compile a design history file (dhf) for ampere meds tech product? Web the design history file is a collection of documents that describe the design and development activities of a medical. Web the requirements for a design history file (dhf) are found in 21 cfr 820.30j: Web freyr provides design history file (dhf) compliance support for medical device manufacturers that includes dhf.