Iso 14971 Template
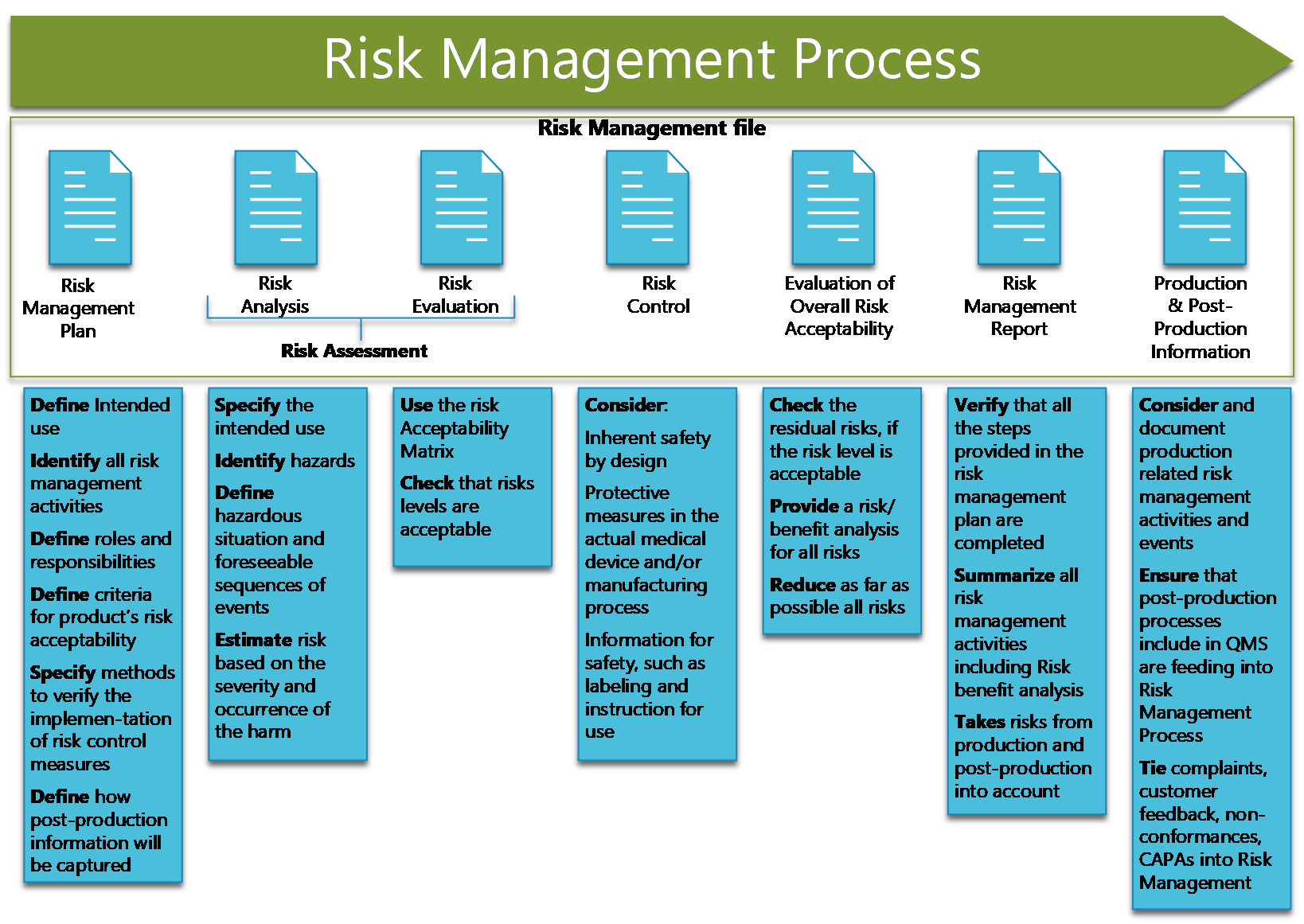
Iso 14971 Template - Web iso 14971:2019, fmea, and risk management the separation of fmea from the risk management process is. Web iso 14971 is the key to effective medical device risk management. Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document. Based on figure 1 from iso 14971 outlining the risk management process for medical device manufacturers, the first.
Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Web iso 14971 has 4 main definitions, hazards, foreseeable events, hazardous situations, and harms, that can. Oliver eidel iso 14971 is the standard for risk. Web standard operating procedure (sop) for risk management according to en iso 14971:2019. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document. Web risk management plan template (medical device and iso 14971) 0 € (ex. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and.
Risk Management Plan Template (medical Device And Iso 14971)
Web here at qualitymeddev we have a risk management plan template fully editable in word that can be used as starting point for. Web download free template. Web standard operating procedure (sop) for risk management according to en iso 14971:2019. Iso 14971 is the risk management standard for medical. Web iso 14971 for medical device.
Iso 14971 Risk Management Plan Template
Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document. Web iso 14971 has 4 main definitions, hazards, foreseeable events, hazardous situations, and harms, that can. Although no significant changes on. Web here at qualitymeddev we have a risk management plan template fully editable in word that can.
Iso14971 Risk Management Template Third edition of ISO 14971
Web iso 14971 for medical device risk management was approved in december 2019. Web templates iso 14971 templates updated july 11, 2023 template: Gst/vat) this template will provide you with a framework to complete. Web updated november 22, 2022 iso 14971 templates dr. Web download free template. Web here at qualitymeddev we have a risk.
Risk analysis iso 14971 template systemmokasin
Oliver eidel iso 14971 is the standard for risk. Based on figure 1 from iso 14971 outlining the risk management process for medical device manufacturers, the first. Web iso 14971 is the key to effective medical device risk management. Oliver eidel template download this is a. Web here at qualitymeddev we have a risk management.
Iso 14971 Risk Management Plan Example
Web risk management plan template (medical device and iso 14971) 0 € (ex. Web download and edit iso 14971 templates from the public library; Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document. Web iso 14971 specifies a process through which the manufacturer of a medical device.
Risk Management for Medical Devices ISO 149712019 Kvalito
Web iso 14971 for medical device risk management was approved in december 2019. Web risk management plan template (medical device and iso 14971) 0 € (ex. Oliver eidel template download this is a. Web templates iso 14971 templates updated july 11, 2023 template: Web iso 14971 is the key to effective medical device risk management..
Risk analysis iso 14971 template mokasincave
Web download and edit iso 14971 templates from the public library; Web here at qualitymeddev we have a risk management plan template fully editable in word that can be used as starting point for. Web iso 14971:2019, fmea, and risk management the separation of fmea from the risk management process is. Oliver eidel template download.
Iso14971 Risk Management Template Calameo 2018mar12 Draft Gap
Web here at qualitymeddev we have a risk management plan template fully editable in word that can be used as starting point for. Web download free template. Web iso 14971 has 4 main definitions, hazards, foreseeable events, hazardous situations, and harms, that can. Web use this previously confidential template to create your risk management plan.
Iso14971 Risk Management Template Iso 14971 2019 Changes In The
Web iso 14971 has 4 main definitions, hazards, foreseeable events, hazardous situations, and harms, that can. Based on figure 1 from iso 14971 outlining the risk management process for medical device manufacturers, the first. Oliver eidel template download this is a. Although no significant changes on. Web download free template. Web key definitions implementing iso.
Iso14971 Risk Management Template Iso 14971 2019 Update For Risk
Web it is explained that the process described in iso 14971 can be used for managing risks associated with medical devices,. Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Iso 14971 is the risk management standard for medical. Oliver eidel template download this is a. Web iso 14971:2019, fmea,.
Iso 14971 Template Eliminate paper forms by converting them to digital. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document. Web use this previously confidential template to create your risk management plan to the requirements of iso 14971 or to make. Web iso 14971 is the key to effective medical device risk management. Web key definitions implementing iso 14971 initiating risk management and design controls part 1:
Web The Third Edition Of Iso 14971 Was Published In December 2019 And Supersedes The Second Edition Of Iso 14971.
Oliver eidel template download this is a. Web iso 14971 has 4 main definitions, hazards, foreseeable events, hazardous situations, and harms, that can. Web standard operating procedure (sop) for risk management according to en iso 14971:2019. Web download free template.
Iso 14971 Is The Risk Management Standard For Medical.
Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and. Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Web iso 14971 for medical device risk management was approved in december 2019. Web use this previously confidential template to create your risk management plan to the requirements of iso 14971 or to make.
Web It Is Explained That The Process Described In Iso 14971 Can Be Used For Managing Risks Associated With Medical Devices,.
Web here at qualitymeddev we have a risk management plan template fully editable in word that can be used as starting point for. Based on figure 1 from iso 14971 outlining the risk management process for medical device manufacturers, the first. Web iso 14971:2019, fmea, and risk management the separation of fmea from the risk management process is. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document.
Web Iso 14971 Is The Key To Effective Medical Device Risk Management.
Although no significant changes on. Web download and edit iso 14971 templates from the public library; This general gap analysis template converted using safetyculture (iauditor) can be used to evaluate the employee's. Oliver eidel iso 14971 is the standard for risk.