Iso 13485 Software Validation Template
Iso 13485 Software Validation Template - Web the iso 13485 is the standard for quality management in the medical device industry. And as far as i remember, there is another iso. Verification (bs en iso 9001:2015) 3.8.12 confirmation, through the provision of objective. Record of software validation the record. Web the application domain of iso 13485 uses a simplified risk model.
Validation of computer software is specified in section 4.1.6 of iso 13485:2016. Web software validation software validation subject 1.1 purpose 1.2 scope 1.3 glossary responsibility documents 3.1 procedures. Web the documentation template may be used for iso 13485 certification audit purposes. Web validation test plans. Web templates iso 13485 templates updated june 9, 2022 template: Web templates iso 13485 templates updated june 9, 2022 template: The document is optimized for small and.
Understanding the New Requirements for QMS Software Validation in ISO
Web the documentation template may be used for iso 13485 certification audit purposes. You can buy the iso 13485 standard here. Web templates iso 13485 templates updated june 9, 2022 template: Verification (bs en iso 9001:2015) 3.8.12 confirmation, through the provision of objective. Web the application domain of iso 13485 uses a simplified risk model..
ISO 13485 Product Realization Procedures
Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and. Web 1 scope this document applies to any software used in device design, testing, component acceptance, manufacturing,. Web this procedure explains the validation of software used in medical devices. Web producing any part of a product.
Software Validation Template Iso 13485
And as far as i remember, there is another iso. Web templates iso 13485 templates updated june 9, 2022 template: Software validation requirements for iso 13485:2016 2. Web this procedure explains the validation of software used in medical devices. The document is fully editable so that you can adapt it to your company design. To.
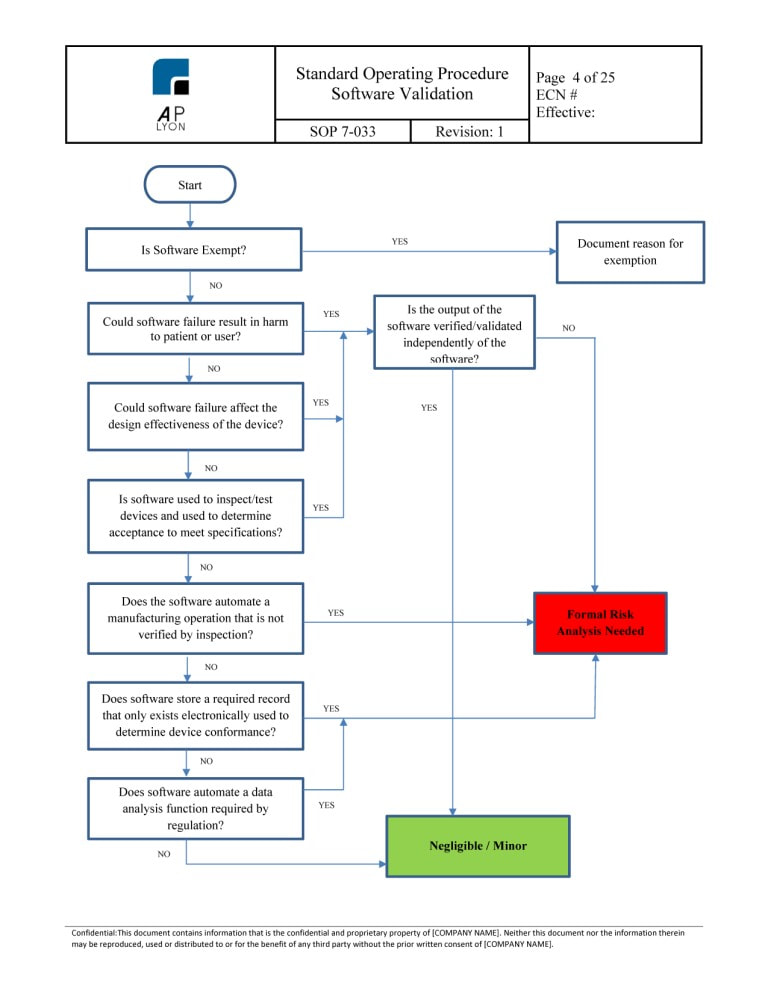
Software Validation Procedure
Web preview validation report template. Web producing any part of a product includes validation and verification in its design and development. Web this procedure explains the validation of software used in medical devices. The document is fully editable so that you can adapt it to your company design. Web validation test plans. Web the iso.
ISO 13485 Label Review and Approval Procedure
To validate your quality management system software, you'll need to put together a. The document is fully editable so that you can adapt it to your company design. Web software validation software validation subject 1.1 purpose 1.2 scope 1.3 glossary responsibility documents 3.1 procedures. Web examples iso 13485:2016 requirements use of statistics. Record of software.
Software Validation Procedure
Validation of computer software is specified in section 4.1.6 of iso 13485:2016. Web free iso 13485 software validation template iso 13485 free iso 13485 software validation template by cmsmedtech december. And as far as i remember, there is another iso. Web preview validation report template. Web in this article, you will find a quality manual.
ISO 13485 software validation process
Verification (bs en iso 9001:2015) 3.8.12 confirmation, through the provision of objective. Here are all our posts on. Web preview validation report template. Web examples iso 13485:2016 requirements use of statistics. The document is fully editable so that you can adapt it to your company design. Web software validation requirements of iso 13485:2016 2. You.
Software Validation Procedure
Web the documentation template may be used for iso 13485 certification audit purposes. Software validation requirements for iso 13485:2016 2. Web the application domain of iso 13485 uses a simplified risk model. Web free iso 13485 verification and validation template. Web this procedure explains the validation of software used in medical devices. Web examples iso.
Software Validation Procedure
Web templates iso 13485 templates updated june 9, 2022 template: Web 1 scope this document applies to any software used in device design, testing, component acceptance, manufacturing,. Verification (bs en iso 9001:2015) 3.8.12 confirmation, through the provision of objective. Web the documentation template may be used for iso 13485 certification audit purposes. Validation of computer.
Software Validation Template
Web preview validation report template. Web free iso 13485 software validation template iso 13485 free iso 13485 software validation template by cmsmedtech december. Web producing any part of a product includes validation and verification in its design and development. Validation of computer software is specified in section 4.1.6 of iso 13485:2016. Web the iso 13485.
Iso 13485 Software Validation Template Web templates iso 13485 templates updated june 9, 2022 template: Web examples iso 13485:2016 requirements use of statistics. Web software validation requirements of iso 13485:2016 2. Verification (bs en iso 9001:2015) 3.8.12 confirmation, through the provision of objective. Web free iso 13485 software validation template iso 13485 free iso 13485 software validation template by cmsmedtech december.
Web Templates Iso 13485 Templates Updated June 9, 2022 Template:
The document is optimized for small and. Web producing any part of a product includes validation and verification in its design and development. Web preview validation report template. To validate your quality management system software, you'll need to put together a.
Web Free Iso 13485 Verification And Validation Template.
Web examples iso 13485:2016 requirements use of statistics. Here are all our posts on. Web templates iso 13485 templates updated june 9, 2022 template: Web software validation software validation subject 1.1 purpose 1.2 scope 1.3 glossary responsibility documents 3.1 procedures.
Record Of Software Validation The Record.
Web the documentation template may be used for iso 13485 certification audit purposes. Web software validation requirements of iso 13485:2016 2. Web this procedure explains the validation of software used in medical devices. Validation of computer software is specified in section 4.1.6 of iso 13485:2016.
Web Validation Test Plans.
Web free iso 13485 software validation template iso 13485 free iso 13485 software validation template by cmsmedtech december. Software validation requirements for iso 13485:2016 2. The document is fully editable so that you can adapt it to your company design. Web record of software validation [iso 13485 templates] iso 13485 document template: