Hydrogen Bond Drawing
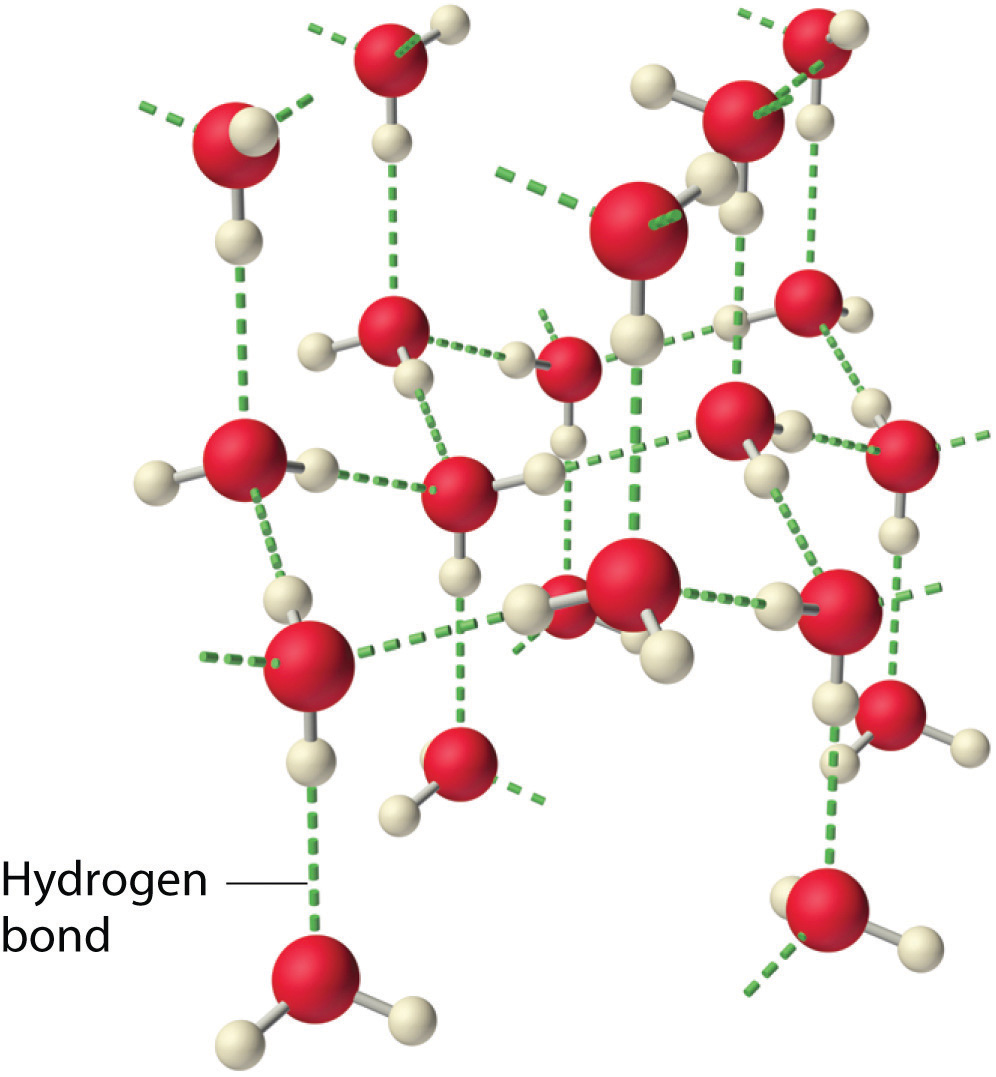
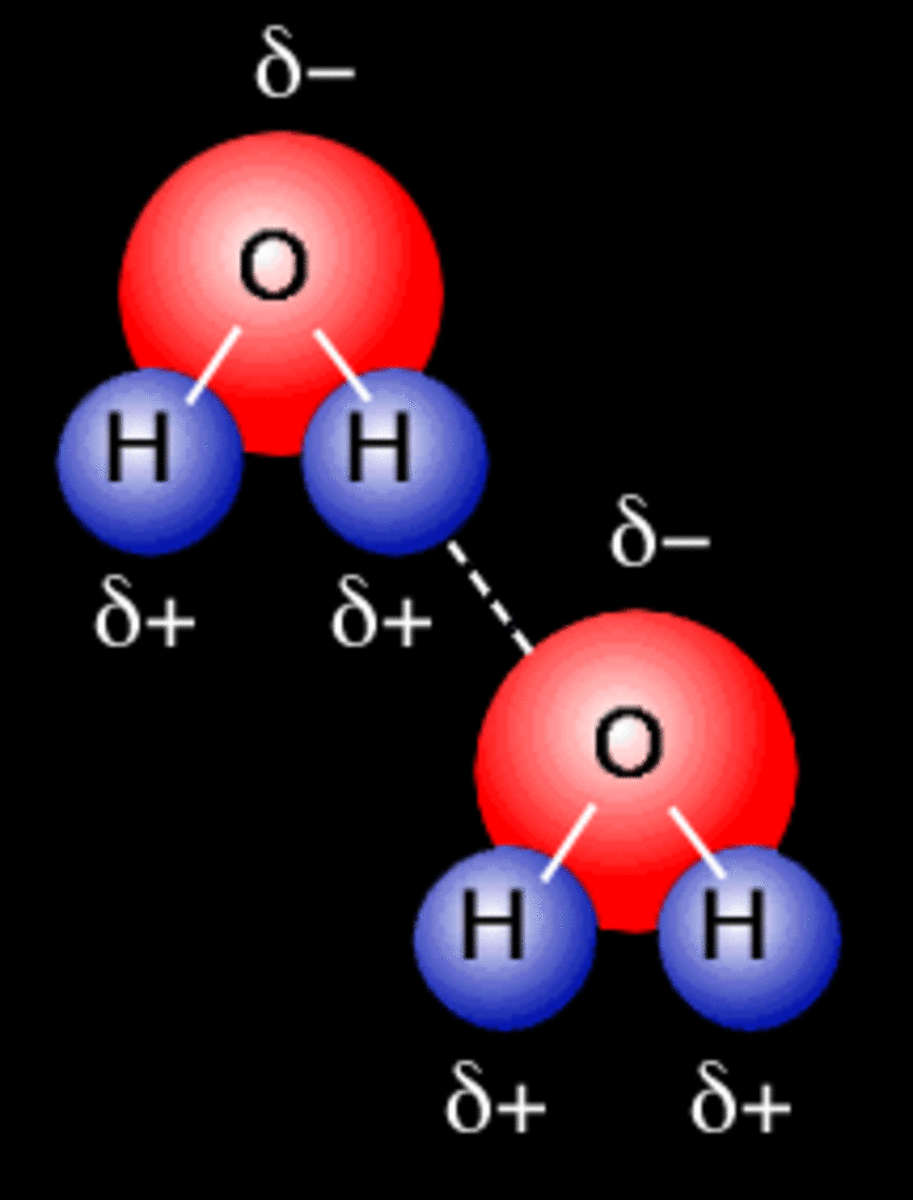
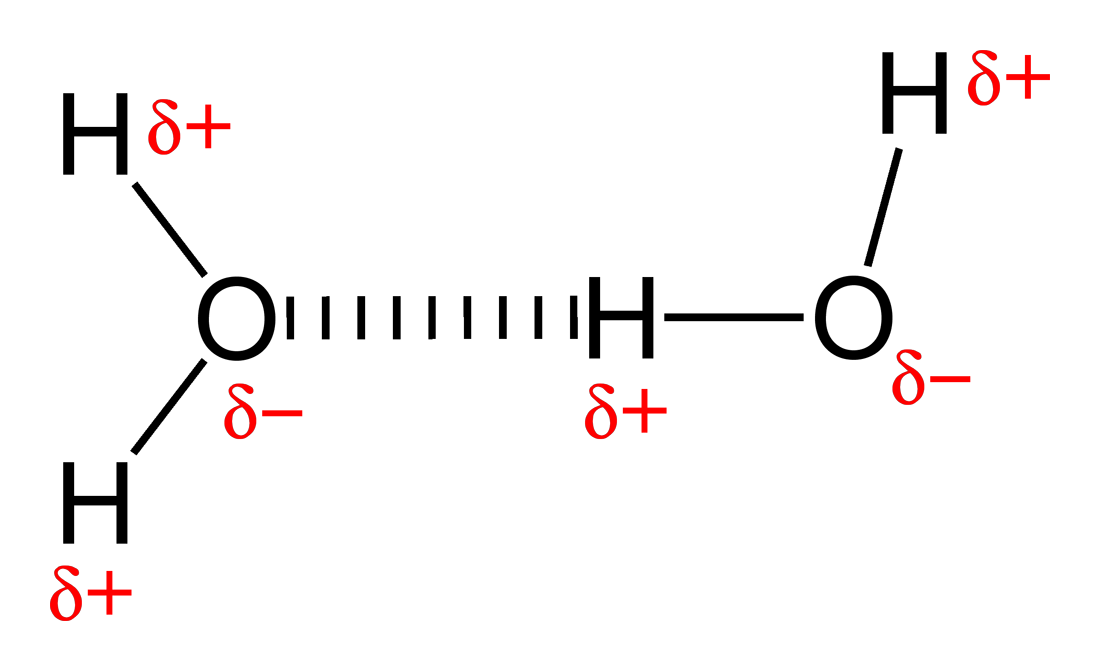
Hydrogen Bond Drawing - For hydrogen bonding to take place the following is needed: Oxygen is highly electronegative, which creates a partial negative charge on one end of the molecule, and a partial positive charge on the other. The hbond representation will draw a dotted line between two atoms if there is a possible hydrogen bond between them. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. For a hydrogen bond to form you need 3 atoms and a lone pair of electrons, it is important that when you are asked to draw a hydrogen bond that the atoms and the lone pair of electrons involved in the hydrogen bond are in a straight line with each other.
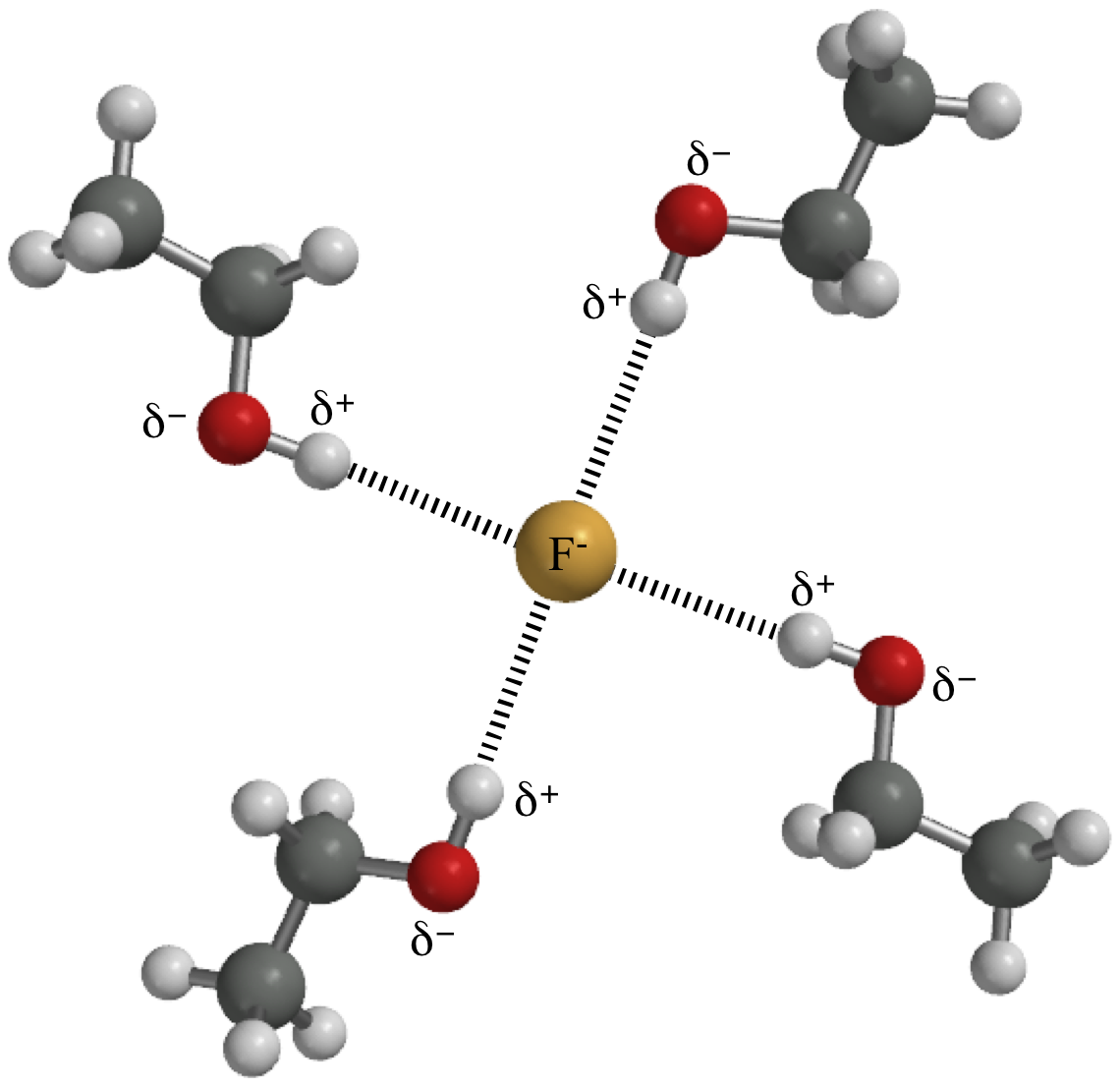
Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Describe the structure, such as it is, of liquid water. Hydrogen bond is an attraction force that formed between 2. It also depicts the uneven electron density distribution in a covalent bond thus it first forms in a single molecule where. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Hydrogen atoms attached to fluorine, oxygen, or nitrogen on one molecule; In methoxymethane, the lone pairs on the oxygen are still there, but the hydrogens aren't sufficiently δ+ for hydrogen bonds to form.
H2O Lewis Structure, Molecular Geometry, and Hybridization
A possible hydrogen bond is defined by the following criteria: Considered individually, hydrogen bonds are much weaker than a single covalent bond, such as a phosphodiester bond. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Web if you were to draw every carbon hydrogen bond in organic.
The Curious Wavefunction A bond by any other name... How the simple
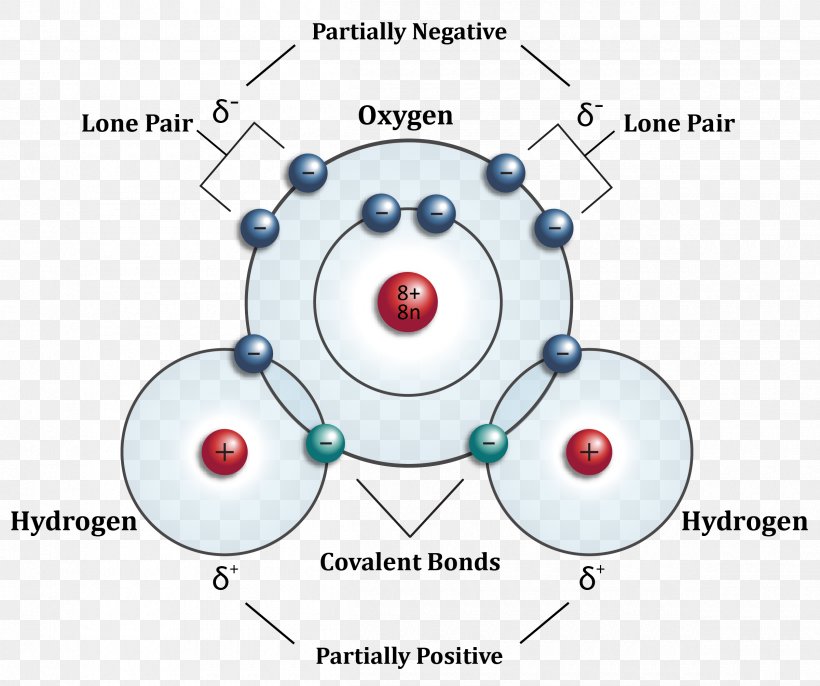
All of the electron pairs—shared and unshared—repel each other. There are two requirements for hydrogen bonding. Hydrogen bonds are especially strong intermolecular forces. For hydrogen bonding to take place the following is needed: Web explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Hydrogen bonds can exist between.
Hydrogen Bonding Definition, Example, Types, Question Embibe
Lone pairs of electrons on another molecule. But, there are so many of them that the two dna polymers are very strongly connected to each other. The hbond representation will draw a dotted line between two atoms if there is a possible hydrogen bond between them. One of the most common mistakes is to write.
Hydrogen Bond Mechanism, Hydrogen Bond in Water, Examples, Videos
But, there are so many of them that the two dna polymers are very strongly connected to each other. Describe the structure, such as it is, of liquid water. There are two requirements for hydrogen bonding. For a hydrogen bond to form you need 3 atoms and a lone pair of electrons, it is important.
[DIAGRAM] Labeled Diagram Of Hydrogen Bonding
Web hydrogen bonds drawing and explanation. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. Connect the atoms to each other with single bonds to form a.
11.5 Hydrogen Bonds Chemistry LibreTexts
Lone pairs of electrons on another molecule. Add enough electrons (dots) to the outer atoms to. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Figure out how many.
Illustrated Glossary of Organic Chemistry Hydrogen bonding
Considered individually, hydrogen bonds are much weaker than a single covalent bond, such as a phosphodiester bond. Web the nucleotides forming each dna strand are connected by noncovalent bonds, called hydrogen bonds. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Hydrogen bonds have strengths ranging from 5.
Hydrogen Bonds — Overview & Examples Expii
Describe the roles of hydrogen bonding in proteins and in dna. Hydrogen bonds are especially strong intermolecular forces. This video shows three examples of drawing for the formation of hydrogen bond. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. Web hydrogen bonds drawing and.
Hydrogen Atom Water Molecule Molecular Orbital Diagram, PNG
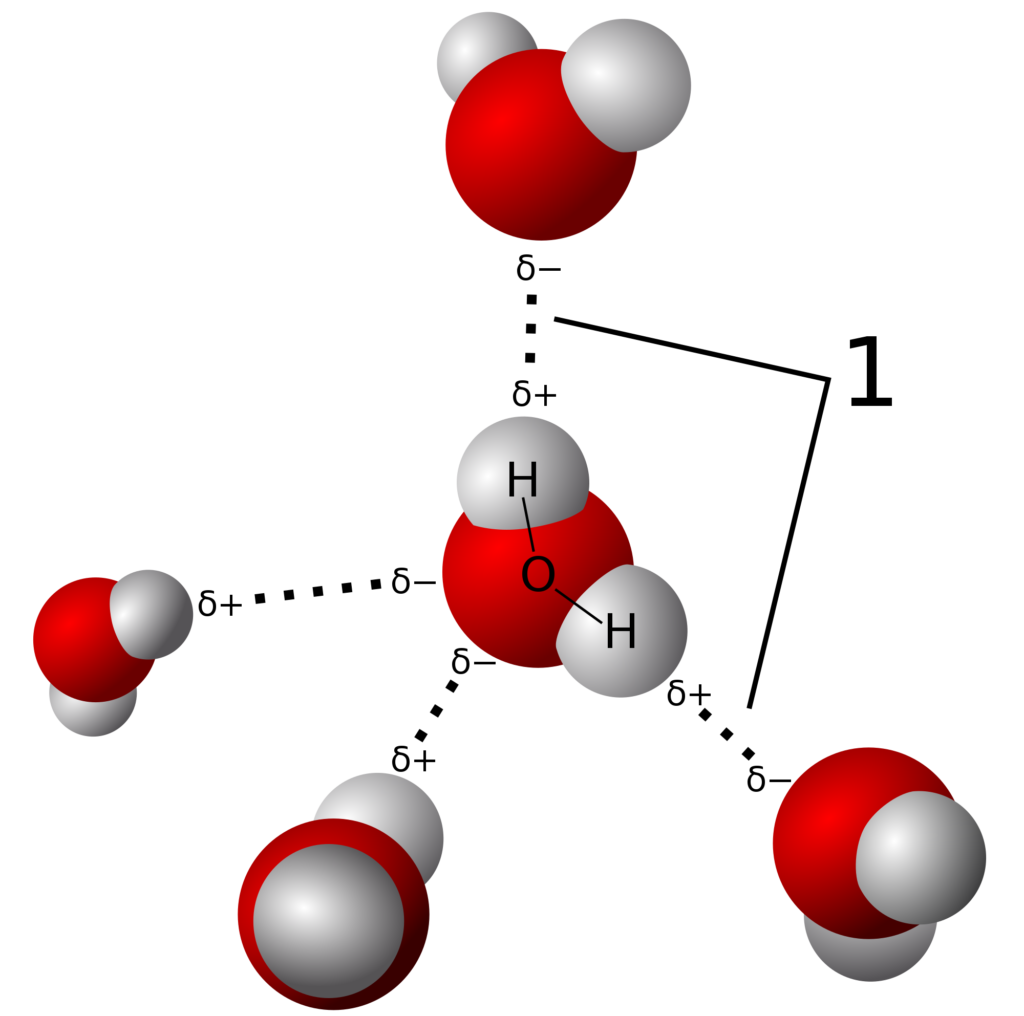
All of the electron pairs—shared and unshared—repel each other. Add enough electrons (dots) to the outer atoms to. Describe the structure, such as it is, of liquid water. The diagram below shows how one hydrogen bond can form in. Oxygen is highly electronegative, which creates a partial negative charge on one end of the molecule,.
Primary and Secondary Bonds Owlcation
How to draw a hidrogen bond or a short contact in chembio3d? Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. This video shows three examples of drawing for the formation of hydrogen bond. Hydrogen bonds are especially strong intermolecular forces. Intermolecular bonds are bonds between.
Hydrogen Bond Drawing Lone pairs of electrons on another molecule. Oxygen is highly electronegative, which creates a partial negative charge on one end of the molecule, and a partial positive charge on the other. A possible hydrogen bond is defined by the following criteria: A dipole exists as a pair, partial positive and partial negative. Describe the structure, such as it is, of liquid water.
This Is Because The Oxygen Atom, In Addition To Forming Bonds With The Hydrogen Atoms, Also Carries Two Pairs Of Unshared Electrons.
Oxygen is highly electronegative, which creates a partial negative charge on one end of the molecule, and a partial positive charge on the other. But, there are so many of them that the two dna polymers are very strongly connected to each other. So, we leave those out in bond line structures. Web the nucleotides forming each dna strand are connected by noncovalent bonds, called hydrogen bonds.
Web How To Draw A Hidrogen Bond Or A Short Contact In Chembio3D?
Describe the roles of hydrogen bonding in proteins and in dna. Hydrogen atoms attached to fluorine, oxygen, or nitrogen on one molecule; Web explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Web step 1:
Web Hydrogen Bonding Is The Strongest Form Of Intermolecular Bonding.
Lone pairs of electrons on another molecule. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. Describe the structure, such as it is, of liquid water. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever.
Carbon Is Still Bonded To These Hydrogens But We're Going To Ignore Them For Our Bond Line Structure.
I need to specify the conformer/stereoisomer by adding. Web bookshelves organic chemistry organic chemistry (morsch et al.) 1: Sketch out structural examples of hydrogen bonding in three small molecules other than h 2 o. The diagram below shows how one hydrogen bond can form in.



![[DIAGRAM] Labeled Diagram Of Hydrogen Bonding](https://i.stack.imgur.com/CzAgU.png)