How To Draw Dipole Arrows
How To Draw Dipole Arrows - Web 1 answer rzp7 · stefan v. The net dipole is the measurable, which is called the dipole. The vector points from positive to negative, on both the molecular (net) dipole moment and. Web transcript like bonds, molecules can also be polar. Web dipole arrows are drawn on lewis structures and point towards the more electronegative atom, since they pull electrons towards them.
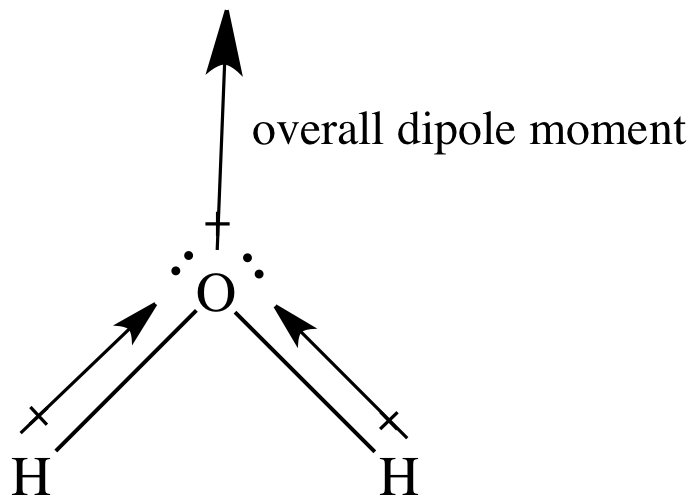
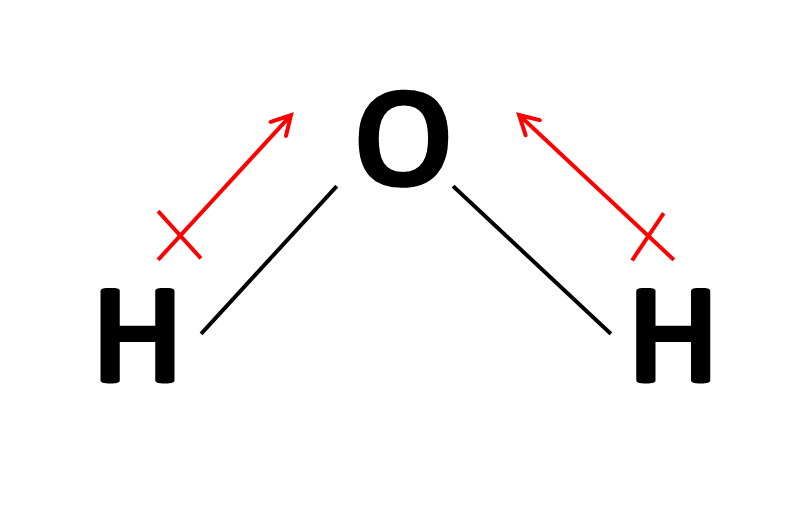
Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Web dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. Web dipole arrows are drawn on lewis structures and point towards the more electronegative atom, since they pull electrons towards them. Web the convention in chemistry is that the arrow representing the dipole moment goes from positive to negative. From in between the hydrogen atoms to the oxygen atom. Physicist tend to use the opposite orientation. Example between o and f, the dipole would point to f.
In a sketch of the bent watermolecule, the head of the two bond dipole
Also learn how to draw a dipole moment arrow to show which side of a molecule is slightly p. Example between o and f, the dipole would point to f. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright..
SOLVED Draw Dipole arrows for all of the polar covalent bonds in the
People keep using center here, and i think students will misinterpret this. The net dipole is the measurable, which is called the dipole. Web 28 i understand that molecular dipoles are electric dipoles. Web mathematically, dipole moment (µ) = charge (q) * distance of separation (r) it is measured in debye units denoted by ‘d’..
How To Draw Overall Dipole Moment DRAWINGS OF LOVE
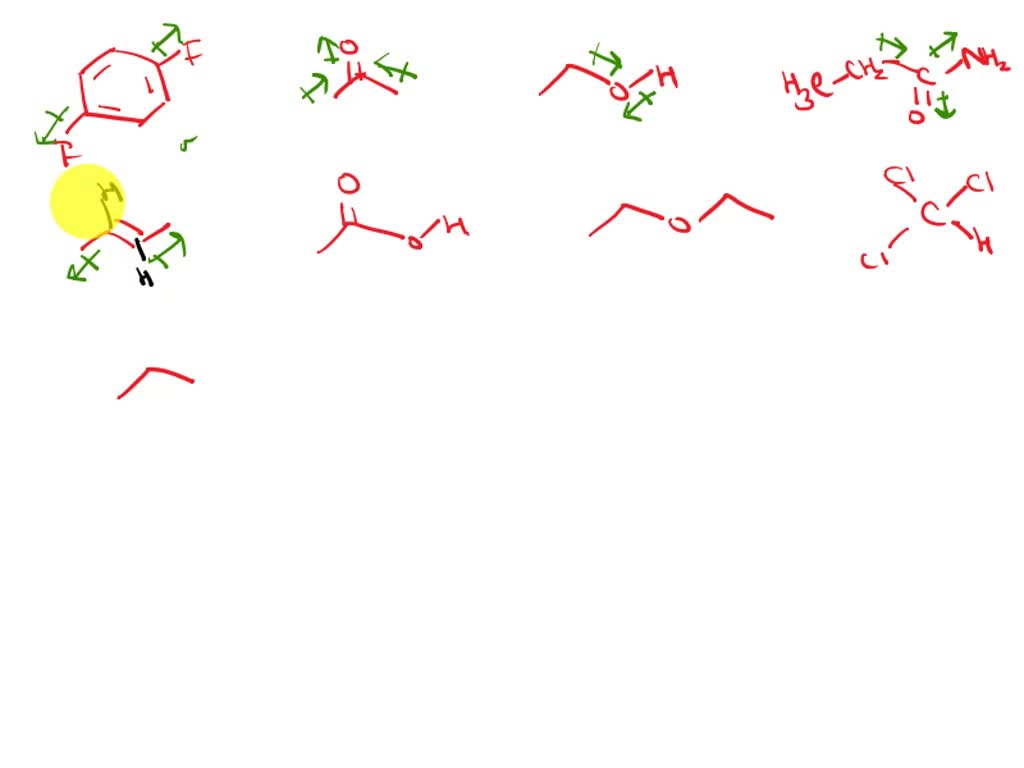
For dipole arrows at an angle, separate them into horizontal and vertical vector components. Web dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. Dipole arrows postby eliana witham 2h » mon nov 30, 2020 7:48 am dipole arrows represent the dipole moment in.
[Solved] how to draw the net dipole arrows for NF3, and which ones are
Thu oct 01, 2020 4:48 am been upvoted: Top eliana witham 2h posts: A small plus sign is drawn on the less electronegative end to indicate the partially positive end of the bond. Dipole arrows postby eliana witham 2h » mon nov 30, 2020 7:48 am dipole arrows represent the dipole moment in a molecule..
Understanding Dipole Arrows YouTube
The more modern way to note a dipole moment is an arrow pointing towards the positive charge. It explains how to indic. Dipole arrows postby eliana witham 2h » mon nov 30, 2020 7:48 am dipole arrows represent the dipole moment in a molecule. The dipole should point to the more electronegative element in a.
How Do You Determine Which Way the Dipole Arrow Points
Web dipole moment arrows. We start by looking at a water molecule: For dipole arrows at an angle, separate them into horizontal and vertical vector components. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge. Using a molecular geometry.
Bond Polarity, Electronegativity and Dipole Moment Chemistry Practice
Step 4) look at the dipoles. A dipole arrow is crossed at the beginning (as in a plus sign) and points in the direction of the greatest electron density. We start by looking at a water molecule: And electric dipole moment vectors point from the negative to the positive charge. Web about press copyright contact.
How do we draw the dipole moment of a water molecule? Socratic
The dipole should point to the more electronegative element in a bond of two elements. The more modern way to note a dipole moment is an arrow pointing towards the positive charge. The bond dipole moment that arises in a chemical bond between two atoms of different electronegativities can be expressed as follows: And electric.
Drawing dipole arrows and determining molecular polarity YouTube
Web dipole moment arrows. Web dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. Dipole arrows point towards the more electronegative element. Example between o and f, the dipole would point to f. The bond dipole moment that arises in a chemical bond between.
[Solved] how to draw the net dipole arrows for H2S, and which ones are
When you draw a dipole moment, the old way of signifying the dipole moment was an arrow pointing towards the negative charge. A small plus sign is drawn on the less electronegative end to indicate the partially positive end of the bond. Top eliana witham 2h posts: Using the cross bow arrow shown below we.
How To Draw Dipole Arrows Thu oct 01, 2020 4:48 am been upvoted: Web dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. Also learn how to draw a dipole moment arrow to show which side of a molecule is slightly p. Web 28 i understand that molecular dipoles are electric dipoles. Web the arrow refers to the direction of the dipole moment.
Web Draw In Dipole Arrows For All Polar Covalent Bonds, Starting The Arrow At The More Electropositive Atom, And Ending At The More Electronegative Atom.
Web dipole moment arrows. The more modern way to note a dipole moment is an arrow pointing towards the positive charge. Web what do they show? Web dipole arrows are drawn on lewis structures and point towards the more electronegative atom, since they pull electrons towards them.
People Keep Using Center Here, And I Think Students Will Misinterpret This.
Web dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. Physicist tend to use the opposite orientation. A small plus sign is drawn on the less electronegative end to indicate the partially positive end of the bond. Also learn how to draw a dipole moment arrow to show which side of a molecule is slightly p.
The Vector Points From Positive To Negative, On Both The Molecular (Net) Dipole Moment And The Individual Bond Dipoles.
From in between the hydrogen atoms to the oxygen atom. Example between o and f, the dipole would point to f. The dipole should point to the more electronegative element in a bond of two elements. Web because of this, the polarization of covalent bonds is typically shown using a special arrow (a dipole arrow) to indicate the direction in which the bond is polarized.
Using The Cross Bow Arrow Shown Below We Can Show That It Has A Net Dipole.
Top eliana witham 2h posts: For dipole arrows at an angle, separate them into horizontal and vertical vector components. This is why in water the dipole arrows are drawn going from hydrogen (low electronegativity) towards oxygen (higher electronegativity). If a polar bond exists then dipole arrows must.