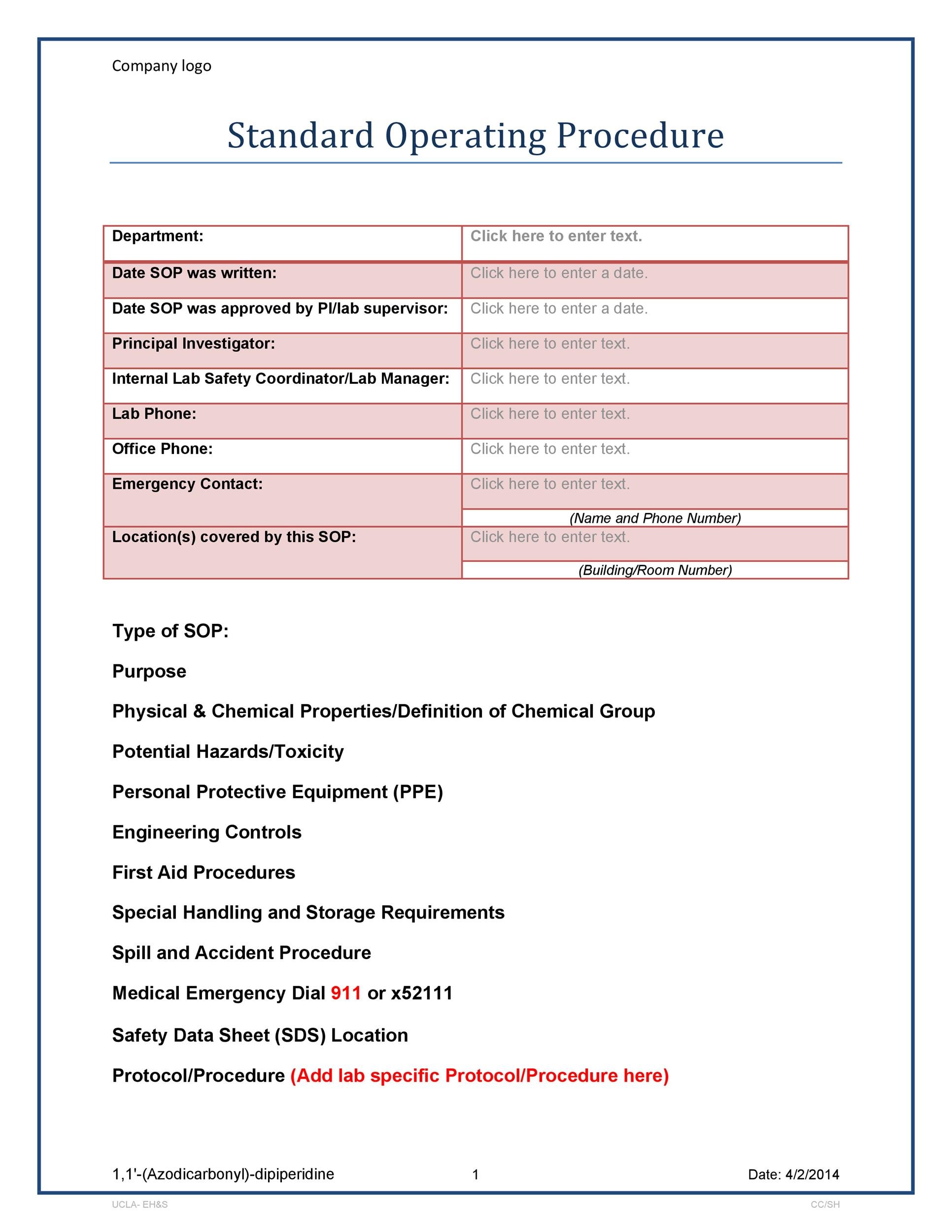
Gmp Sop Template Free
Gmp Sop Template Free - Gmprocedures are time saving sop. Web 240 sops, 197 gmp manuals, 64 templates, 30 training modules, 167 forms. Group ph100 quality unit responsibility sop templates. Web calibration of dissolution test apparatus (usp apparatus 1 and 2) $150.00 $129.00. Web standard operating procedures template.
• all gmp inspections requested by the chmp/cvmp during the evaluation phase of initial applications for. These sop's template will provide a foundation for compliance with fda,. Class f class 5, 6, 7 : Web our products and services we are your resource centre for gmp document templates for life science, fmcg & critical facilities: These sop's will provide a foundation for. Get two high quality standard operating procedures pharmaceutical quality documents for free for regulatory guidance, compliance with fda | eu. Free download, sop, standard operating procedure.
Q040111 DOCUMENT CHANGE CONTROL SOP GMP Templates
• all gmp inspections requested by the chmp/cvmp during the evaluation phase of initial applications for. Free download, sop, standard operating procedure. Web download free gmp forms. Web a set of 68 sops, policies and templates that provides a quality system for any holding and distribution company. These sop's will provide a foundation for. Web.
INTERNAL QUALITY AUDIT SOP Template MD11 GMP, QSR & ISO Comp
Group ph100 quality unit responsibility sop templates. Web 240 sops, 197 gmp manuals, 64 templates, 30 training modules, 167 forms. Web our products and services we are your resource centre for gmp document templates for life science, fmcg & critical facilities: Web say hello to standard operating procedure templates. Web get your gmp sop template.
37 Best Standard Operating Procedure (SOP) Templates
Web standard operating procedures template. Web sop templates for medical device and pharmaceutical manufacturers. Forms belongs to quality assurance and validation standard operating procedures (sop) which can be. Web get your gmp sop template documents at one place. • all gmp inspections requested by the chmp/cvmp during the evaluation phase of initial applications for. +.
DESIGN CONTROLS SOP Template MD20 GMP, QSR & ISO Compliance
These sop's will provide a foundation for. Web a set of 68 sops, policies and templates that provides a quality system for any holding and distribution company. Web download free gmp forms. Web download free template. Web an sop template is a document used to create an organization’s standard operating procedures and avoid common. E.g.,.
GOOD DOCUMENTATION PRACTICES SOP Template MD25 GMP, QSR & ISO CP
Forms belongs to quality assurance and validation standard operating procedures (sop) which can be. Web an sop template is a document used to create an organization’s standard operating procedures and avoid common. These sop's template will provide a foundation for compliance with fda,. A gmp site walkthrough checklist is a tool used in conducting a.
It Department Sop Template PDF Template
Free download, sop, standard operating procedure. Web standard operating procedures template. Web an sop template is a document used to create an organization’s standard operating procedures and avoid common. Web sop templates for medical device and pharmaceutical manufacturers. Are designed to get your project moving quickly and efficiently. Web say hello to standard operating procedure.
GMP Protocols and Reports GMP Docs
+ german vat when applicable. These sop's template will provide a foundation for compliance with fda,. Additional documents included each month. Get two high quality standard operating procedures pharmaceutical quality documents for free for regulatory guidance, compliance with fda | eu. Read along to access 10 free sop templates for clickup, google. Web 240 sops,.
DOCUMENT & CHANGE CONTROLS SOP Template MD22 GMP, QSR & ISO CP
Web sop templates for medical device and pharmaceutical manufacturers. Web a manufacturing standard operating procedure (sop) is a set of documented instructions created to help workers perform routine. Group ph100 quality unit responsibility sop templates. Web say hello to standard operating procedure templates. Web calibration of dissolution test apparatus (usp apparatus 1 and 2) $150.00.
QUALITY INVESTIGATIONS SOP Template MD35 GMP, QSR & ISO Comp
Web sop templates for medical device and pharmaceutical manufacturers. Web calibration of dissolution test apparatus (usp apparatus 1 and 2) $150.00 $129.00. Web download free template. Web our products and services we are your resource centre for gmp document templates for life science, fmcg & critical facilities: Get two high quality standard operating procedures pharmaceutical.
organic system plan template Saferbrowser Image Search Results
Web a set of 68 sops, policies and templates that provides a quality system for any holding and distribution company. Web say hello to standard operating procedure templates. E.g., 10 kg to 250 kg. Web sop templates for medical device and pharmaceutical manufacturers. Web purchase the most important quality good manufacturing practice (gmp) document templates.
Gmp Sop Template Free • all gmp inspections requested by the chmp/cvmp during the evaluation phase of initial applications for. Free download, sop, standard operating procedure. Web download free template. Web say hello to standard operating procedure templates. Web purchase the most important quality good manufacturing practice (gmp) document templates for active pharmaceutical ingredients (api) and drug.
Are Designed To Get Your Project Moving Quickly And Efficiently.
Web sop templates for medical device and pharmaceutical manufacturers. Group ph100 quality unit responsibility sop templates. Web our products and services we are your resource centre for gmp document templates for life science, fmcg & critical facilities: Get two high quality standard operating procedures pharmaceutical quality documents for free for regulatory guidance, compliance with fda | eu.
Forms Belongs To Quality Assurance And Validation Standard Operating Procedures (Sop) Which Can Be.
Web download free template. • all gmp inspections requested by the chmp/cvmp during the evaluation phase of initial applications for. Web a manufacturing standard operating procedure (sop) is a set of documented instructions created to help workers perform routine. Gmprocedures are time saving sop.
Additional Documents Included Each Month.
+ german vat when applicable. Web a set of 68 sops, policies and templates that provides a quality system for any holding and distribution company. These sop's template will provide a foundation for compliance with fda,. Web calibration of dissolution test apparatus (usp apparatus 1 and 2) $150.00 $129.00.
Free Download, Sop, Standard Operating Procedure.
Web an sop template is a document used to create an organization’s standard operating procedures and avoid common. Web 240 sops, 197 gmp manuals, 64 templates, 30 training modules, 167 forms. Web standard operating procedures template. Web download free gmp forms.