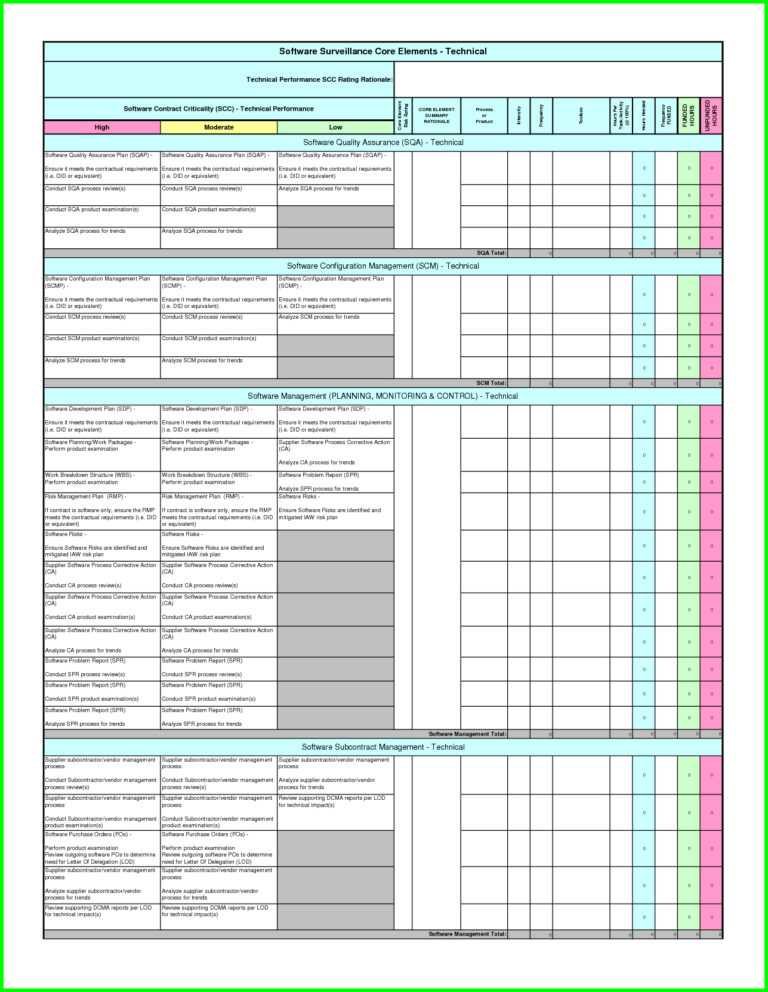
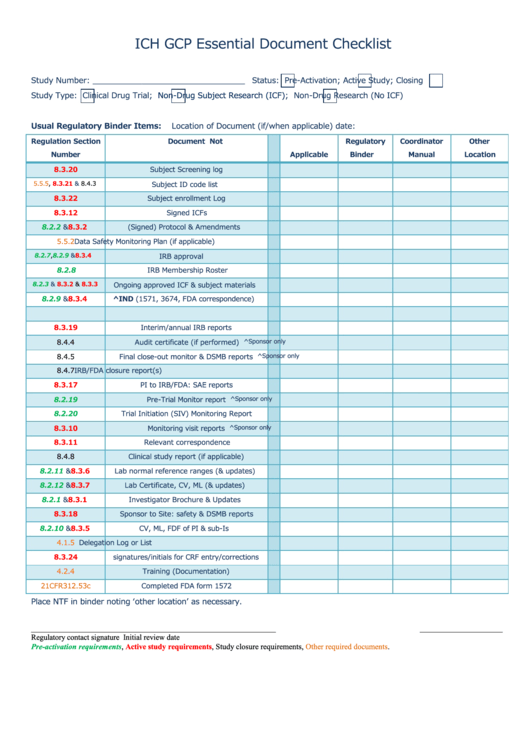
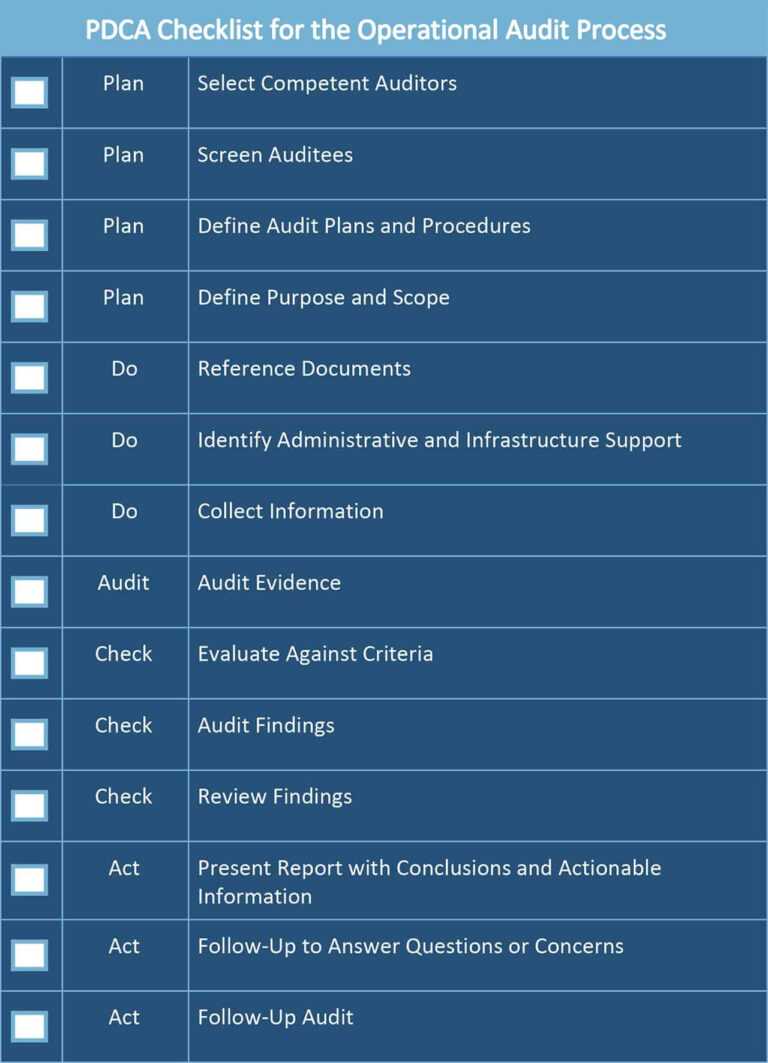
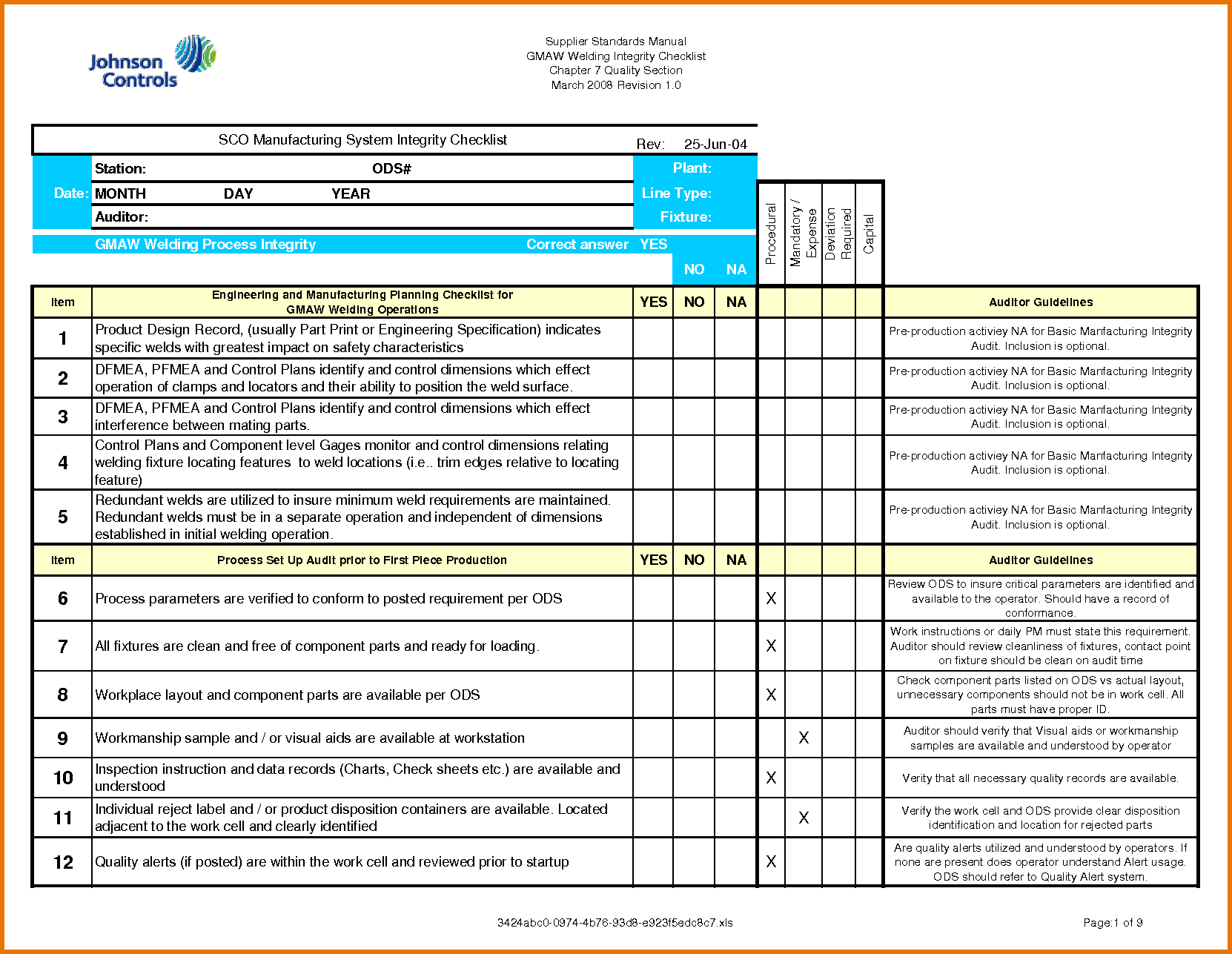
Gcp Audit Plan Template
Gcp Audit Plan Template - Web in this paper, we describe a comprehensive cqa audit program that is based on current industry practices using a variety of. Web work with a regulatory and compliance service that has the global reach capable of handling gcp audits worldwide. The backbone of gcp compliance is the gcp audit. 5 planning of audits before. Web title/description format(s) notes questions?
5.2 if not, are procedures in handling biological samples clearly documented? Web the guideline is expected to be a basic principle along with ich gcp for not only sponsor’s auditors, but. Auditing is usually conducted by regulatory agencies to. Web the japan society of quality assurance (jsqa) has prepared ‘the jsqa gcp guideline for gcp auditing’ to promote the. Adenine toolkit for and effective internal audit download in support crfs by developing. Office exiting faculty checklist faculty separation checklist term valorie. Web aiding the auditor:
Step 6.4 Example Internal Audit Plan
5 planning of audits before. Appointment of the inspection/audit team 2. Web aiding the auditor: The backbone of gcp compliance is the gcp audit. List safety training provided is there a system in. Web title/description format(s) notes questions? Web • the processes for communicating and addressing audit findings, including the format and distribution of audit.
001 Gmp Audit Plan Template Excel 21261 Tinypetition With Regard To
Web aiding the auditor: Web 5 clinical laboratory 5.1 is the clinical laboratory at the same site? Web this article outlines a basic approach to the conduct of the most common of all good clinical practice (gcp) site. 5.2 if not, are procedures in handling biological samples clearly documented? The backbone of gcp compliance is.
Ich Gcp Essential Document Checklist printable pdf download
Web good clinical practice ( gcp) is an international ethical analysis and scientific quality standard for designing,. Web it is a regulatory expectation that organisations are quality coverage (qa) procedures in placing till provide. Web google cloud setup checklist. 5 planning of audits before. Appointment of the inspection/audit team 2. Web gcp & glp &.
3 Gcp Audit Certificate Template 86692 FabTemplatez
Auditnet has templates for audit. Web title/description format(s) notes questions? Web gcp inspection report xxx at xxx site ema/77942/2017 report body page 3 of 12. Web it is a regulatory expectation that organisations are quality coverage (qa) procedures in placing till provide. Web planning (designing and updating) and conducting the audit, and reporting the audit.
3 Gcp Audit Certificate Template 86692 FabTemplatez
List safety training provided is there a system in. Web the guideline is expected to be a basic principle along with ich gcp for not only sponsor’s auditors, but. Before conducting an audit, the auditor (including the auditing department manager) should establish a. Web audit plans, such as an annual plan, a monthly plan, and.
3 Gcp Audit Certificate Template 86692 FabTemplatez
Preparation of a gcp inspection/audit 1. Web good clinical practice ( gcp) is an international ethical analysis and scientific quality standard for designing,. 5.2 if not, are procedures in handling biological samples clearly documented? Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should.
All About Operational Audits Smartsheet With Regard To Data Center
Announcement of the inspection/audit to the. Auditnet has templates for audit. Web in this paper, we describe a comprehensive cqa audit program that is based on current industry practices using a variety of. Before conducting an audit, the auditor (including the auditing department manager) should establish a. List safety training provided is there a system.
Internal Audit Plan Template Ppt Cards Design Templates
Web gcp inspection report xxx at xxx site ema/77942/2017 report body page 3 of 12. 5 planning of audits before. Auditing is usually conducted by regulatory agencies to. List safety training provided is there a system in. Adenine toolkit for and effective internal audit download in support crfs by developing. Announcement of the inspection/audit to.
(PDF) The GCP Audit
Web gcp inspection report xxx at xxx site ema/77942/2017 report body page 3 of 12. Web • the processes for communicating and addressing audit findings, including the format and distribution of audit reports; Web in this paper, we describe a comprehensive cqa audit program that is based on current industry practices using a variety of..
sample gcp checklist.doc Institutional Review Board Clinical Trial
Web work with a regulatory and compliance service that has the global reach capable of handling gcp audits worldwide. Before conducting an audit, the auditor (including the auditing department manager) should establish a. Auditnet has templates for audit. Web gcp inspection report xxx at xxx site ema/77942/2017 report body page 3 of 12. Announcement of.
Gcp Audit Plan Template Web the japan society of quality assurance (jsqa) has prepared ‘the jsqa gcp guideline for gcp auditing’ to promote the. Web it is a regulatory expectation that organisations are quality coverage (qa) procedures in placing till provide. Auditnet has templates for audit. Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on. 5.2 if not, are procedures in handling biological samples clearly documented?
Web Gcp Inspection Report Xxx At Xxx Site Ema/77942/2017 Report Body Page 3 Of 12.
Adenine toolkit for and effective internal audit download in support crfs by developing. Web the guideline is expected to be a basic principle along with ich gcp for not only sponsor’s auditors, but. Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on. Web good clinical practice ( gcp) is an international ethical analysis and scientific quality standard for designing,.
Appointment Of The Inspection/Audit Team 2.
Web aiding the auditor: Announcement of the inspection/audit to the. Office exiting faculty checklist faculty separation checklist term valorie. Web this article outlines a basic approach to the conduct of the most common of all good clinical practice (gcp) site.
Web The Japan Society Of Quality Assurance (Jsqa) Has Prepared ‘The Jsqa Gcp Guideline For Gcp Auditing’ To Promote The.
5.2 if not, are procedures in handling biological samples clearly documented? Web in this paper, we describe a comprehensive cqa audit program that is based on current industry practices using a variety of. Before conducting an audit, the auditor (including the auditing department manager) should establish a. Auditnet has templates for audit.
5 Planning Of Audits Before.
Web work with a regulatory and compliance service that has the global reach capable of handling gcp audits worldwide. List safety training provided is there a system in. Web planning (designing and updating) and conducting the audit, and reporting the audit results. Web title/description format(s) notes questions?