Drawing The Reaction Energy Diagram Of A Catalyzed Reaction
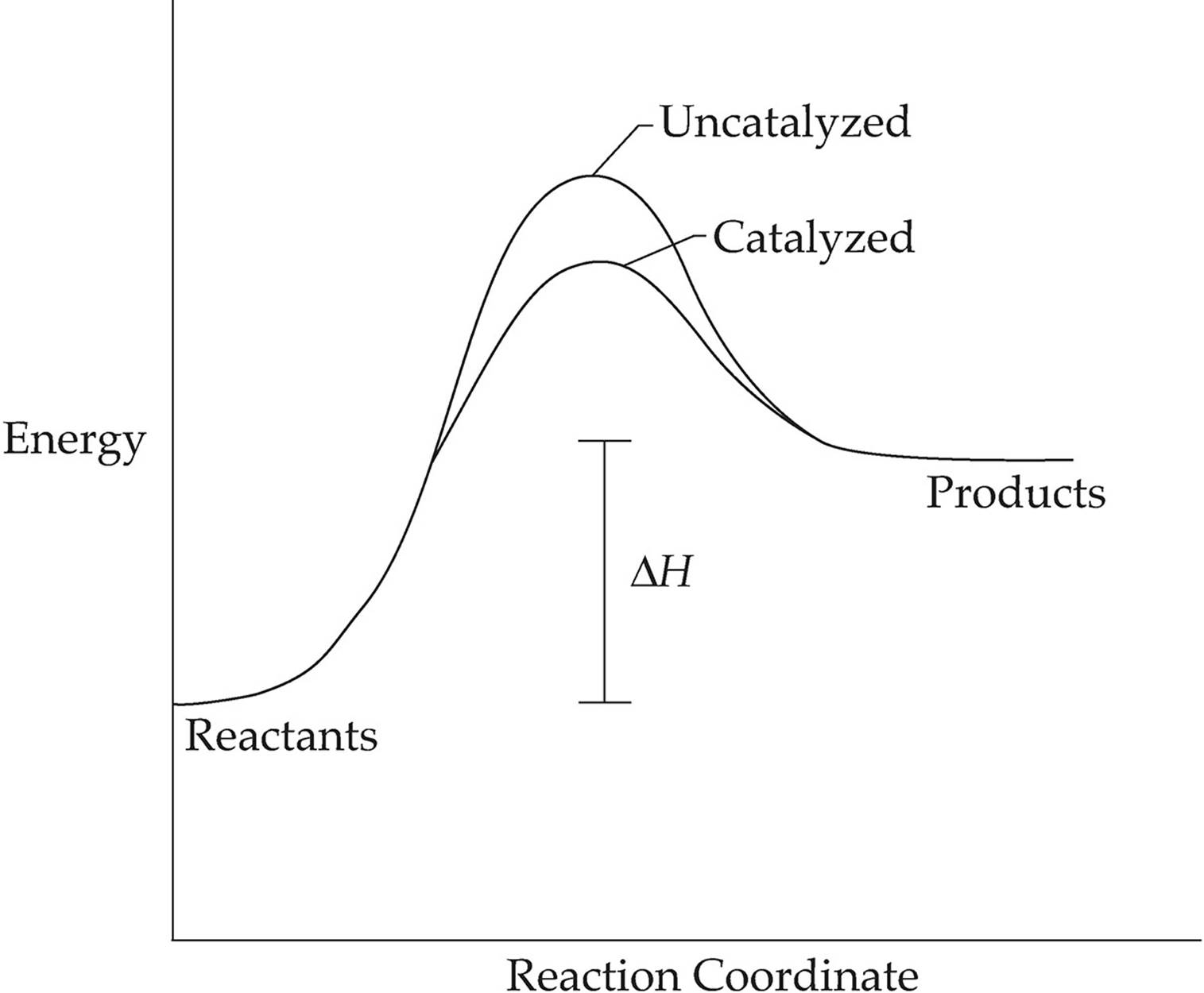
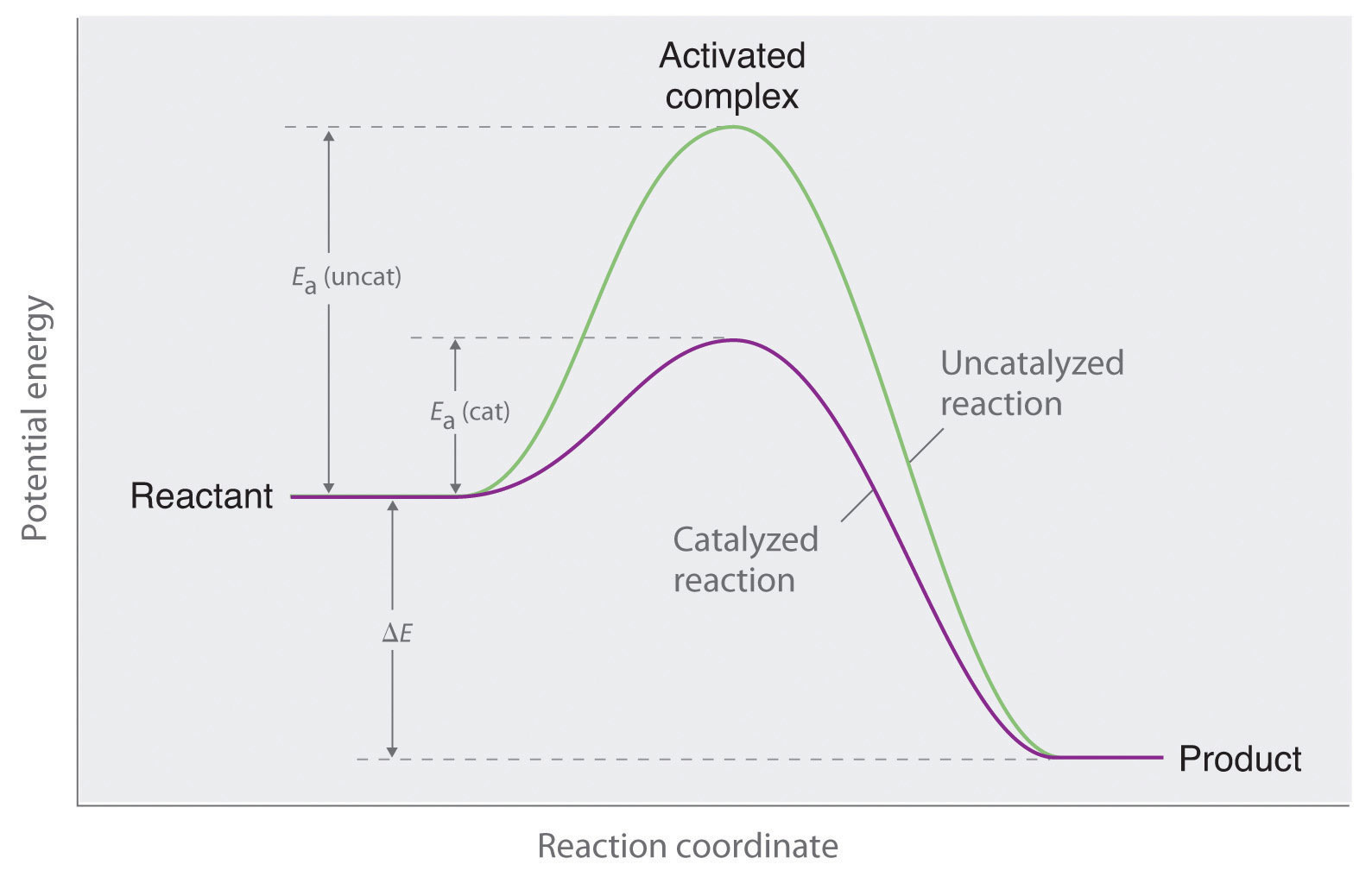
Drawing The Reaction Energy Diagram Of A Catalyzed Reaction - Using reaction diagrams to compare catalyzed reactions the two reaction diagrams here represent the same reaction: Web potential energy diagram of catalyzed and uncatalyzed reactions. The catalyst does not affect the energy of the reactants or. A catalyst like iodine can be used to provide an alternate pathway for the reaction with a much lower activation energy of approximately 118 kj/mol (figures 17.15 “catalyzed. It also shows the effect of a catalyst on the forward and reverse activation energy.
A catalyst like iodine can be used to provide an alternate pathway for the reaction with a much lower activation energy of approximately 118 kj/mol (figures 17.15 “catalyzed. How do molecules have to be arranged and how much energy do they have to collide with? Web d37.2 catalysts and reaction mechanisms. The two reaction diagrams here represent the same reaction: Because the sketches are only qualitative, the energies in them don't have to be exact. Determine the energies of the reactants and products and the reaction enthalpy from the labeled diagram above,. Web drawing the reaction energy diagram of a catalyzed reaction lauren sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst.
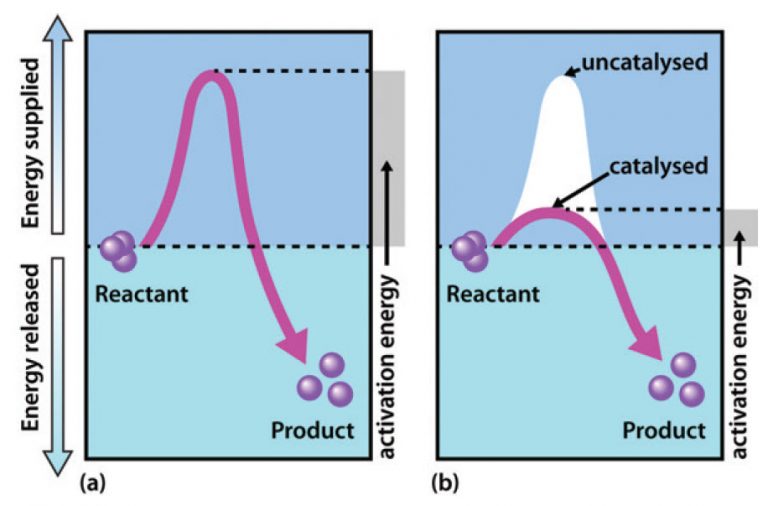
A catalyst speeds up a reaction by providing the reactants with an
What does the orange line represent? Web reaction diagrams for catalyzed reactions. Web drawing the reaction energy diagram of a catalyzed reaction lauren sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Web potential energy diagram of catalyzed and uncatalyzed reactions. Solution a catalyst does not affect the energy.
Catalysis Fundamentals Chemical Engineering Page 1
The two reaction diagrams here represent the same reaction: Web this chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Web potential energy diagram of catalyzed and uncatalyzed reactions. The only effect of the catalyst is to lower the activation energy of the reaction. Determine what the activation energy could be..
Effect of catalyst on energy diagram profile. Download Scientific Diagram
Web the following diagram shows an energy diagram for the reaction between carbon dioxide and water to form carbonic acid. Lowering the activation energy of a reaction by a catalyst. Solution a catalyst does not affect the energy of reactant or product, so those aspects of the diagrams can be ignored; A catalyst increases the.
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry
Web identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Estimate the activation energy for each process, and identify which one involves a catalyst. One without a catalyst and one with a catalyst. The only effect of the catalyst is to lower the activation energy of.
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts
Web it's time to learn a little more about a chemical reaction. Because the sketches are only qualitative, the energies in them don't have to be exact. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Solution a catalyst.
Section 184 Catalysis
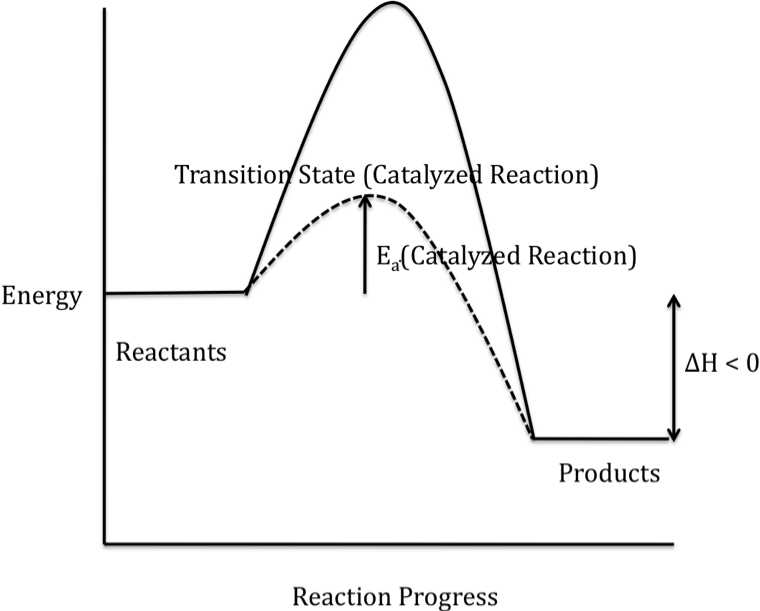
In the energy diagram for an exothermic reaction, the potential energy of the products is lower than that of the reactants.this indicates that energy has been released from the system, as the reactants have converted to products. Web identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed.
Catalysts in 21st Century Energy
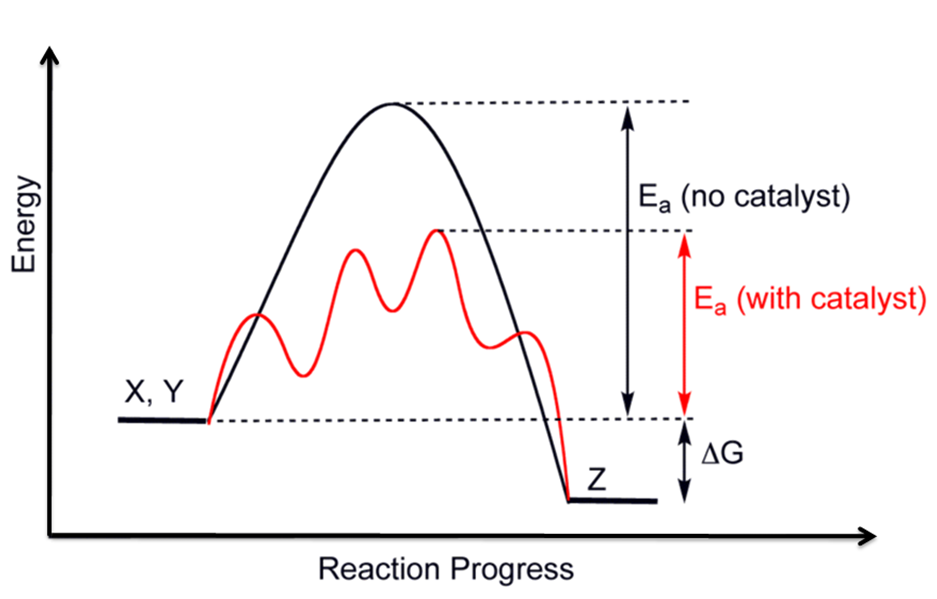
What does the orange line represent? The reaction with catalyst is indicated with a blue line, and the uncatalyzed reaction is indicated with a red line. One without a catalyst and one with a catalyst. A catalyzed mechanism must involve at least two steps, one where the catalyst interacts with a reactant to form an..
Energy Diagram Catalyzed Vs Uncatalyzed Reaction
Draw the transition state of a reaction. One without a catalyst and one with a catalyst. Analyzing the potential energy diagram of a regular/uncatalyzed and a catalyzed (adding a catalyst) reaction. Web the two reaction diagrams below represent the same reaction: Web d37.2 catalysts and reaction mechanisms. The only effect of the catalyst is to.
Energy Diagram Catalyzed Vs Uncatalyzed Reaction
Assume the uncatalyzed reaction is endothermic note: Web draw reaction energy diagrams from the thermodynamic and kinetic data/information. Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed. Draw the transition state of a reaction. Determine the energies of the reactants and products and the reaction enthalpy from.
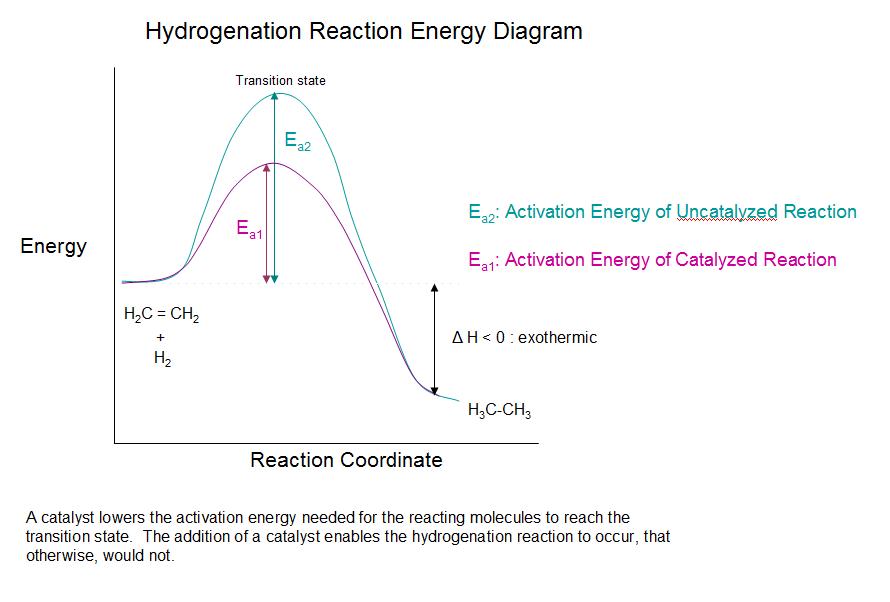
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts
The two reaction diagrams here represent the same reaction: What does the orange line represent? One without a catalyst and one with a catalyst. Web reaction diagrams for catalyzed reactions. Determine the energies of the reactants and products and the reaction enthalpy from the labeled diagram above,. Web figure 14.7.1 14.7. A catalyst increases the.
Drawing The Reaction Energy Diagram Of A Catalyzed Reaction One without a catalyst and one with a catalyst. Web in an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘reaction coordinate’, tracing from left to right the progress of the reaction from starting compounds to final products. Web this chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Web identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Show solution check your learning
The Catalyst Does Not Affect The Energy Of The Reactants Or Products (And Thus Does Not Affect Δ E ).
Web this chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Web reaction diagrams for catalyzed reactions. One without a catalyst and one with a catalyst. What does the orange line represent?
Web About Press Copyright Contact Us Creators Advertise Developers Terms Privacy Policy & Safety How Youtube Works Test New Features Nfl Sunday Ticket Press Copyright.
Web drawing the reaction energy diagram of a catalyzed reaction lauren sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. The only effect of the catalyst is to lower the activation energy of the reaction. Web identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: The reaction with catalyst is indicated with a blue line, and the uncatalyzed reaction is indicated with a red line.
The Two Reaction Diagrams Here Represent The Same Reaction:
Web d37.2 catalysts and reaction mechanisms. One without a catalyst and one with a catalyst. A catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower activation energy than for the uncatalyzed reaction. Use a reaction energy diagram to discuss transition states, ea, intermediates & rate determining step.
You May Recall From General Chemistry That It Is Often Convenient To Describe Chemical Reactions With Energy Diagrams.
In this figure, two graphs are shown. Determine the activation energy the activation energy is the energy of the transition state minus the reactant. Determine the energies of the reactants and products and the reaction enthalpy from the labeled diagram above,. It also shows the effect of a catalyst on the forward and reverse activation energy.