Draw The Main Lewis Structure Of Nof
Draw The Main Lewis Structure Of Nof - With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Clo3 each o atom is bonded to the cl. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Web step 1: Web draw the main lewis structure of nof.
Clo3 each o atom is bonded to the cl. Web in the lewis structure for nof there are a total of 18 valence electrons. For nitrogen (group 15 element), number of valence. Web in this task, we need to draw the main lewis structure of n o f \ce{nof} nof using dot notation for nonbonding electrons and bonds for bonding electrons. Web jamesripley report flag outlined final answer: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Now counting the number of valence electrons in the molecule:
Solved Part B Draw the main Lewis structure of NOF Draw
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of bef2. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Web draw the main lewis structure of nof. Web have you ever wondered how to draw the main lewis structure of nof? Include all lone.
Draw The Main Lewis Structure Of Nof Fotodtp
Web science chemistry chemistry questions and answers draw the lewis structure for nof nitrogen is the central atom draw the molecule by placing atoms on the grid and connecting them with bonds. Web step 1: Now counting the number of valence electrons in the molecule: Draw nonbonding electrons using the dot notation and bonding electrons.
Solved Part B Draw the main Lewis structure of NOF Draw
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of bef2. Clo3 each o atom is bonded to the cl. Web introduction draw the lewis structure of nof (nitrosyl fluoride) chemistnate 248k subscribers subscribe 4.5k views 2 years ago nof is actually onf, since nitrogen has a higher bonding..
NOF Lewis Structure How to Draw the Lewis Structure for NOF YouTube
Part a determine the number of bonding electrons and the number of nonbonding electrons in the structure of co2. Web jamesripley report flag outlined final answer: Web draw the main lewis structure of nof. Thereafter, the valence electrons of all the three atoms. For the nof structure use the periodic table to find the total.
Draw the main lewis structure of nof. draw nonbonding electrons using
Lewis structure is a simplified graphic presentation showing the valence electrons around atoms bonded together to make a molecule. The lewis structure of nof is drawn by determining the total valence electrons, arranging the atoms with the least electronegative in the center, depicting single bonds between the central and surrounding atoms, and distributing the remaining.
draw the main lewis structure of nofnof. darnelllemmings
Part a determine the number of bonding electrons and the number of nonbonding electrons in the structure of co2. Calculate the total number of valence electrons. Thereafter, the valence electrons of all the three atoms. Lewis structure is a simplified graphic presentation showing the valence electrons around atoms bonded together to make a molecule. Web.
Solved Part B Draw the main Lewis structure of NOF. Draw
Thereafter, the valence electrons of all the three atoms. Web in the lewis structure for nof there are a total of 18 valence electrons. Web the structure on the right is the lewis electron structure, or lewis structure, for h 2 o. Figure out how many electrons the molecule must have, based on the number.
Main Lewis Structure Of Nof
Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Web the total number of valence electrons available for drawing the nitrosyl fluoride (nof) lewis. Here, the given molecule is nof. For nitrogen (group 15 element), number of valence. Thereafter, the valence electrons of all the three atoms. The following procedure will.
How to draw lewis structures for NOF in 60s! Dr K shorts YouTube
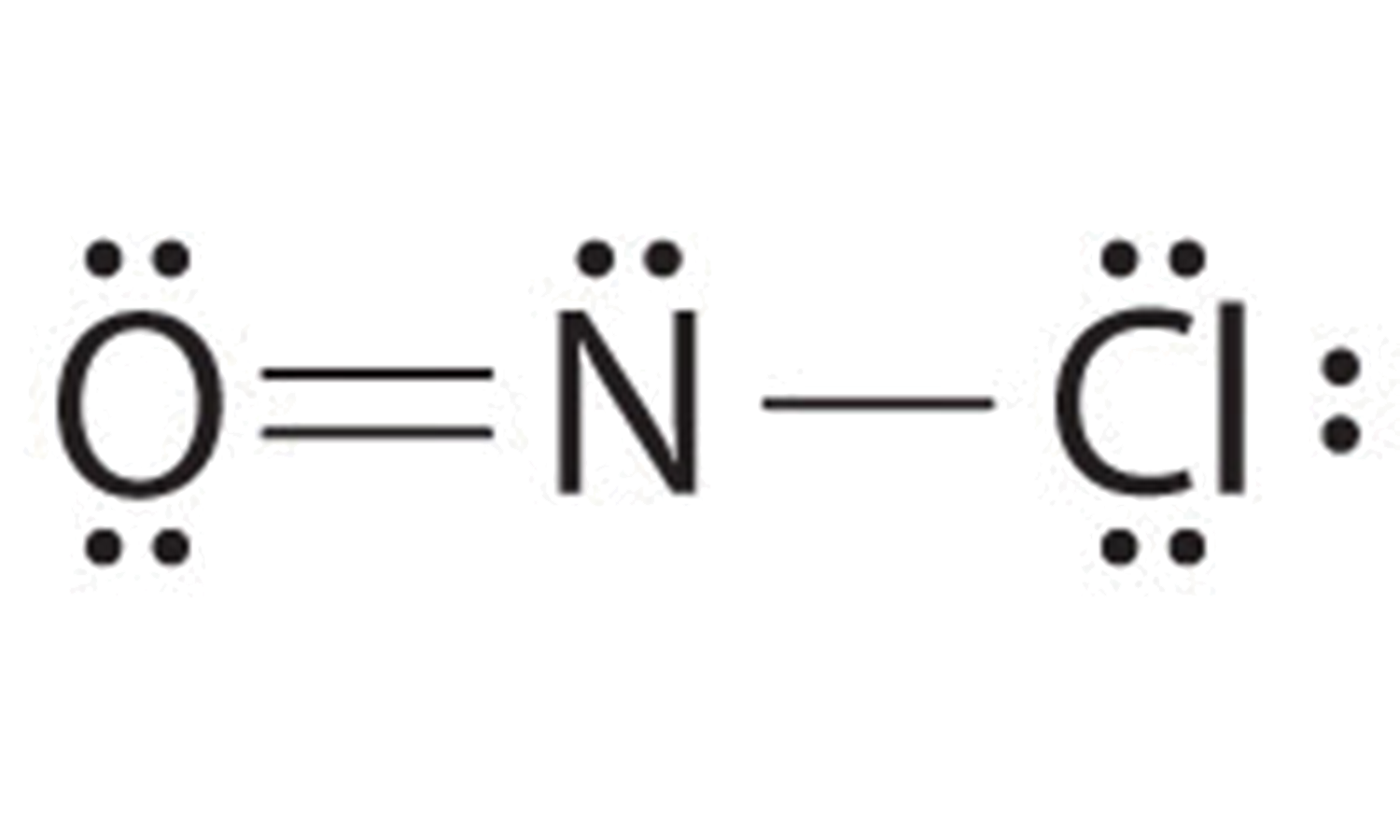
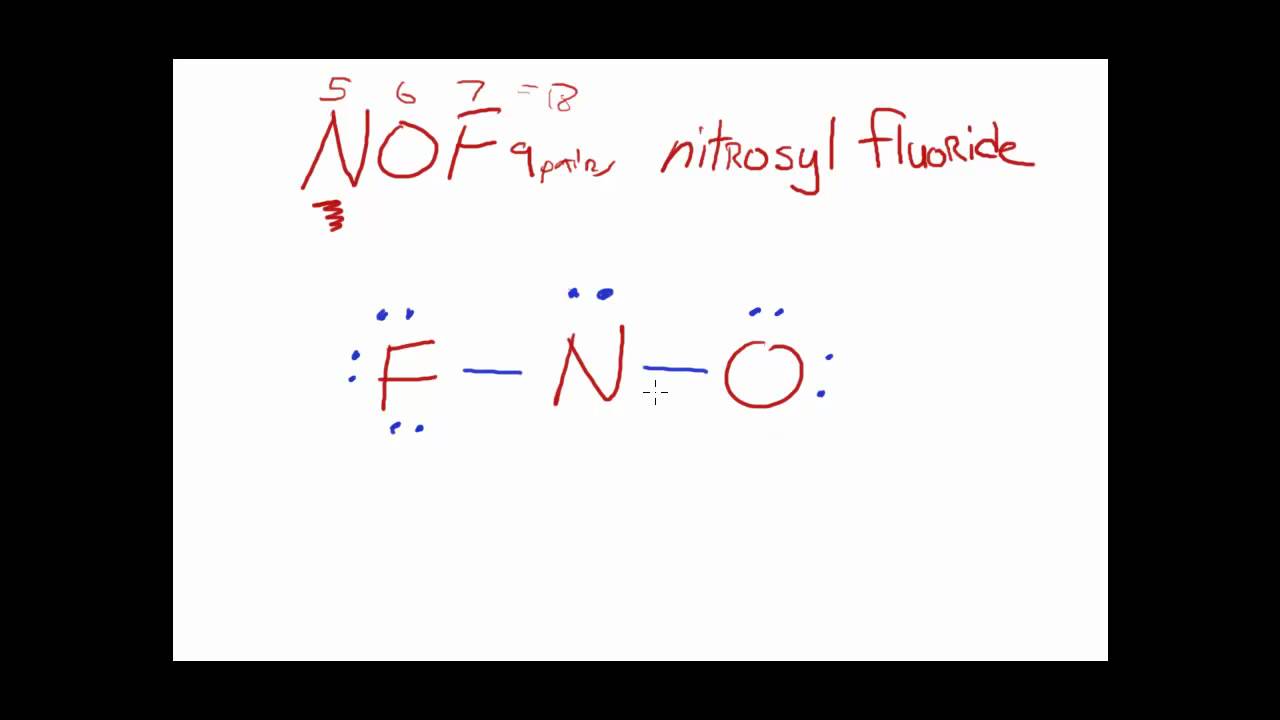
Web the lewis structure of nof contains one double bond and one single bond, with nitrogen in the center, and oxygen and fluorine on either side. Web 6 steps to draw the lewis structure of nof step #1: Here, the given molecule is nof. The oxygen atom has two lone pairs, the nitrogen atom has.
Structure and Geometry The NOF example YouTube
Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Web the structure on the right is the lewis electron structure, or lewis structure, for h 2 o. Web definition and purpose the main purpose of lewis structure s is to show the arrangement of valence electrons around atoms in a molecule.
Draw The Main Lewis Structure Of Nof Web watch on steps of drawing nof lewis structure step 1: Determine the number of bonding electrons and the number of nonbonding electrons in the structure of bef2. Web use these steps to correctly draw the nof lewis structure: Web definition and purpose the main purpose of lewis structure s is to show the arrangement of valence electrons around atoms in a molecule or compound. Now counting the number of valence electrons in the molecule:
Nitrogen, On The Periodic Table, Is In Group 5 Or 15, So It Has 5 Valence Electrons.
Web have you ever wondered how to draw the main lewis structure of nof? Web in the lewis structure for nof there are a total of 18 valence electrons. Web the final lewis structure of nof is: The oxygen atom has two lone pairs, the nitrogen atom has one lone pair, and the fluorine atom has three lone pairs.
Calculate The Total Number Of Valence Electrons.
Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Include all lone pairs of electrons. Web use these steps to correctly draw the nof lewis structure: Determine the number of bonding electrons and the number of nonbonding electrons in the structure of bef2.
Web Introduction Draw The Lewis Structure Of Nof (Nitrosyl Fluoride) Chemistnate 248K Subscribers Subscribe 4.5K Views 2 Years Ago Nof Is Actually Onf, Since Nitrogen Has A Higher Bonding.
For nitrogen (group 15 element), number of valence. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web definition and purpose the main purpose of lewis structure s is to show the arrangement of valence electrons around atoms in a molecule or compound. Web watch on steps of drawing nof lewis structure step 1:
Web Jamesripley Report Flag Outlined Final Answer:
With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Well, you’re in luck because in this article, we will show you exactly how to do it step by step. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the total number of valence electrons available for drawing the nitrosyl fluoride (nof) lewis.