Draw The Lewis Structure Of Ncl3 . Include Lone Pairs
Draw The Lewis Structure Of Ncl3 . Include Lone Pairs - Web lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis structure. 2) the lone pairs are shown in purple. Web science chemistry chemistry questions and answers draw the lewis structure of ncl3. Web there is a lone pair attached to the nitrogen atom, and this fulfills the octet requirements of the entire molecule. Web the electron pair being shared by the atoms is called a bonding pair ;
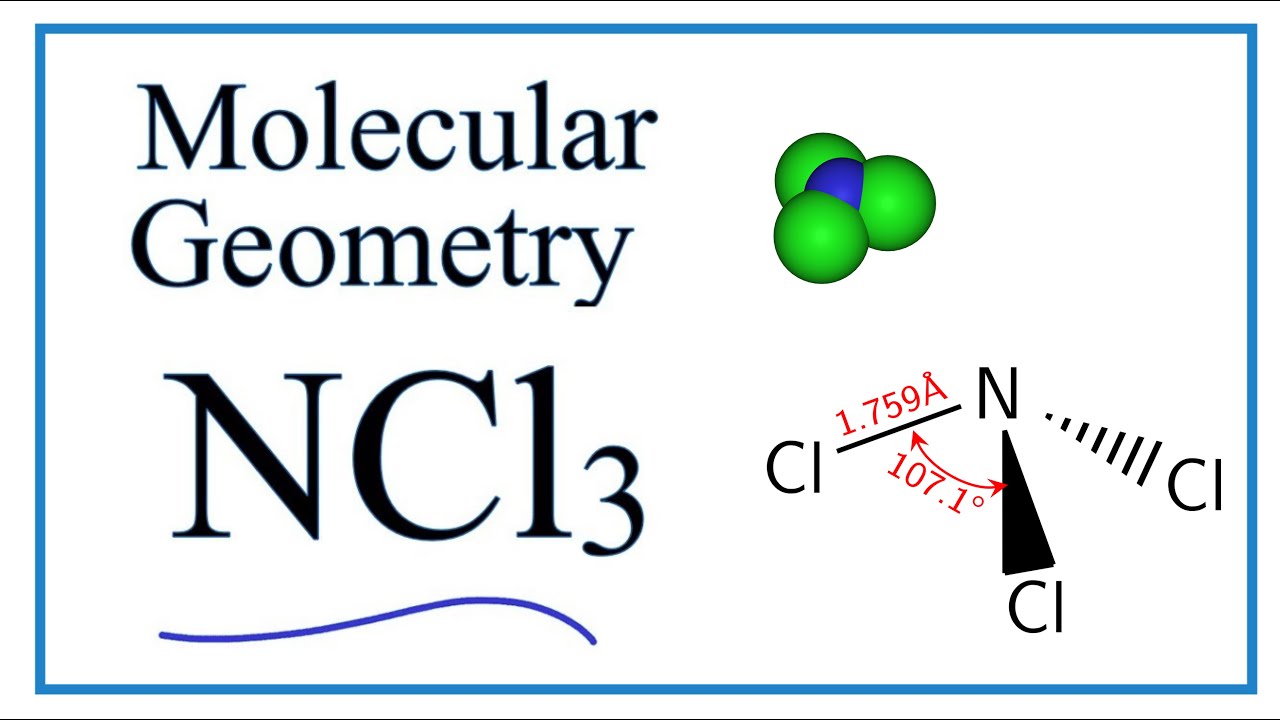
Ncl3 has a lewis structure that is similar to nf3. Draw the lewis structure of the following molecule. Include lone pairs ncl3 this problem has been solved! The lewis structure of ncl3 contains three single bonds, with nitrogen in the center, and three chlorines on either side. Also, there are no charges on atoms in ncl3. Let’s draw and understand this lewis dot structure. The nitrogen atom (n) is at the center and it is surrounded by 3 chlorine atoms (cl).
draw the lewis structure of ncl3 . include lone pairs. tannerringuette
We have to draw the lewis structure of so2 in this question. #1 draw a rough skeleton structure first, determine the total number of valence. Basic concepts of covalent bonding Web there is a lone pair attached to the nitrogen atom, and this fulfills the octet requirements of the entire molecule. The distribution of electrons.
draw the lewis structure of ncl3 . include lone pairs. howtowearcrocs
How to sketch the lewis structure of ncl3: Three pairs will be used in the chemical bonds between the n and cl and one pair of electrons will be unbonded. We have to draw the lewis structure of so2 in this question. If both electrons in a covalent bond come from the same atom, the.
Lewis Structure Ncl3
Include lone pairs ncl3 this problem has been solved! Draw the lewis structure of ncl3. Web science chemistry chemistry questions and answers draw the lewis structure of ncl3. Web lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis.
How to draw NCl3 Lewis Structure? 4
The atom present as a single entity is considered the central atom. There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom. Web science chemistry chemistry questions and answers draw the lewis structure of ncl3. Web chemistry chemistry questions and answers draw the lewis structure of ncl3. 4) the.
NCl3 Lewis Structure and Molecular Geometry Geometry YouTube
The nitrogen atom has 1 lone pair and all the three chlorine atoms have 3 lone pairs. Lone pairs are not involved in covalent bonding. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below. Web lewis structure of ncl3 contains three single bonds between the nitrogen (n).
Draw The Lewis Structure Of Ncl3 Include Lone Pairs
If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. Web chemistry chemistry questions and answers draw the lewis structure of ncl3. The distribution of electrons around individual atoms in a structure is depicted in a lewis diagram. How to sketch the lewis structure of.
SOLVED Draw the Lewis structure of NCl3. Include lone pairs.
How to sketch the lewis structure of ncl3: Web chemistry 101a topic f: Hence, nitrogen is the central atom. The nitrogen atom has 1 lone pair and all the three chlorine atoms have 3 lone pairs. Draw the lewis structure of the following molecule. Web lone pairs, unpaired electrons, and single, double, or triple bonds.
draw the lewis structure of the following molecule include lone pairs
2) the lone pairs are shown in purple. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 9 this problem has been solved! Three pairs will be used in the chemical bonds between the n and cl and one pair of electrons will be unbonded. Include lone pairs.
[Solved] Draw the lewis dot structure for NCl3. Using the VESPER, give
If so, feel free to rate me ath. Three pairs will be used in the chemical bonds between the n and cl and one pair of electrons will be unbonded. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 4) the nitrogen atoms are shown in blue. Web.
NCl3 Molecular Geometry / Shape and Bond Angles YouTube
Draw the lewis structure for co. 2) the lone pairs are shown in purple. 4) the nitrogen atoms are shown in blue. Web chemistry chemistry questions and answers draw the lewis structure of ncl3. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web steps here’s how you.
Draw The Lewis Structure Of Ncl3 . Include Lone Pairs Most structures—especially those containing second row elements—obey the octet rule, in which every atom (except h) is surrounded by eight electrons. We have to draw the lewis structure of so2 in this question. Web chemistry 101a topic f: The lewis structure of ncl3 contains three single bonds, with nitrogen in the center, and three chlorines on either side. Draw the lewis structure for co.
Web Chemistry Chemistry Questions And Answers Draw The Lewis Structure Of The Following Molecule.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below. Web chemistry chemistry questions and answers draw the lewis structure of ncl3. 9 this problem has been solved!
Draw The Molecule By Placing Atoms On The Grid And Connecting Them With Bonds.
The total number of lone pairs of electrons is a. There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom. The nitrogen atom has 1 lone pair and all the three chlorine atoms have 3 lone pairs. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond.
Draw The Lewis Structure Of Ncl3.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. All valence electrons have been used, and the structural arrangement is now stable. Moreover, the element with the lowest electronegativity value becomes the central atom as it is required to make the maximum number of bonds. Lewis structure of ncl3 can be drawn by using valence electrons of nitrogen and chlorine atoms.
Also, There Are No Charges On Atoms In Ncl3.
#1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a closer look at each step mentioned above. Ncl3 has a lewis structure that is similar to nf3. Draw the lewis structure of ncl3. One nitrogen atom is in the middle, with three chlorine atoms equally distributed around it.