Draw The Lewis Structure Of Cof2 . Include Lone Pairs
Draw The Lewis Structure Of Cof2 . Include Lone Pairs - Web this problem has been solved! Draw the lewis structure of cof2. Web to properly draw the cof 2 lewis structure, follow these steps: Find the total valence electrons in cof2 molecule in order to find the total valence electrons in a cof2 molecule, first of all you should know the valence electrons present in carbon atom, oxygen atom as well as fluorine atom. Web science chemistry chemistry questions and answers draw the lewis structures of the following molecules.
Of2 co2 this problem has been solved! Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Sif4 secl2 cof2 (c is central) draw structure. There are three violations to the octet rule: Search for the total number of valence electrons already available in a single carbonyl fluoride atom: Web watch on steps of drawing cof2 lewis structure step 1: Web watch on contents how to draw lewis structure of cof2?
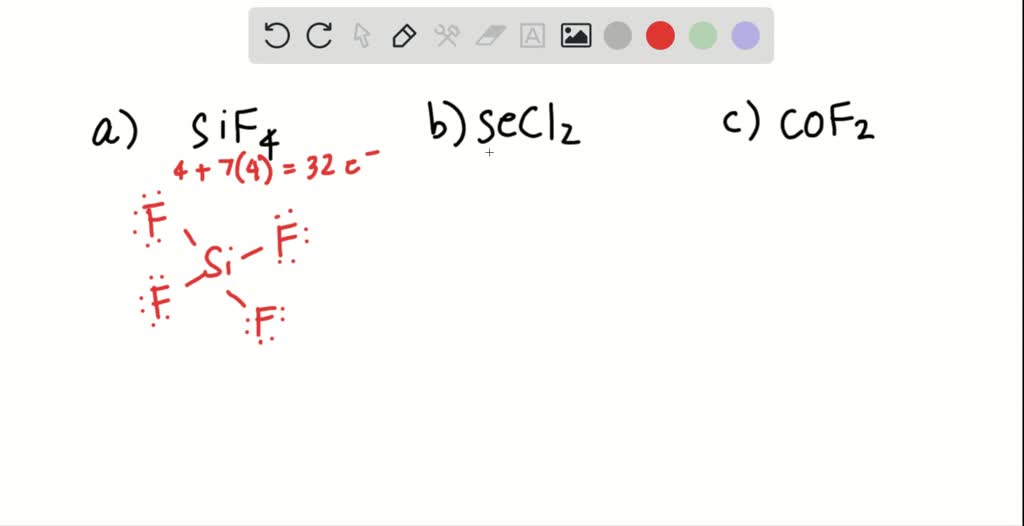
SOLVED Draw a Lewis structure for (a) SiF4; (b) SeCl2; (c) COF2 (C is
Sif4 secl2 cof2 (c is central) this problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Search for the total number of valence electrons already available in a single carbonyl fluoride atom: Draw the molecule by placing atoms on the grid and connecting them.
How to Draw a Lewis Structure
Include all lone pair electrons in your structure. Web chemistry questions and answers. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web watch on steps of drawing cof2 lewis structure step 1: It is 24 as 4 are coming from the.
Lewis Dot Structures How To Calculate The Number of Lone Pairs Using
There are 2 steps to solve this one. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. In the lewis structure for cof 2 there are a total of 24 valence electrons..
Solved Draw the Lewis structure of the following molecule.
Most structures—especially those containing second row elements—obey the octet rule, in which every atom (except h) is surrounded by eight electrons. Web science chemistry chemistry questions and answers draw the lewis structures of the following molecules. It is 24 as 4 are coming from the carbon atom, 6 are coming from the oxygen atom and.
Cof2 Lewis Structure Molecular Geometry Hybridization And Polarity
Also, helium is shown in group 8a, but it only has two valence electrons. Draw a lewis structure for the following. Draw the lewis structure of co2−3.co32−. Web watch on steps of drawing cof2 lewis structure step 1: Sif4 secl2 cof2 (c is central) draw structure. #1 draw skeleton #2 show chemical bond #3 mark.
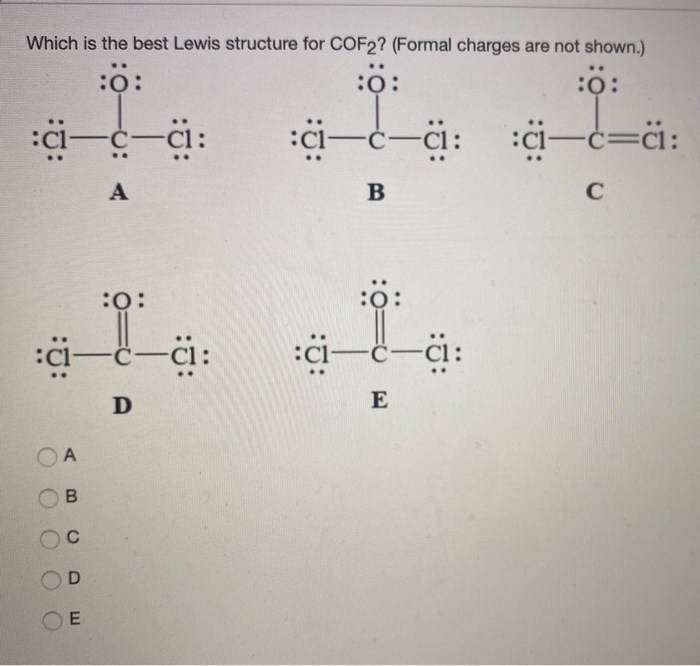
Solved Which is the best Lewis structure for COF2? (Formal
Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web to properly draw the cof 2 lewis structure, follow these steps: Let’s draw and understand this lewis dot structure step by step. You'll need to form a double bond between the carbon and oxygen to complete the.
Draw the Lewis structure (including all lone pair electrons and any
In the lewis structure for cof 2 there are a total of 24 valence electrons. It is represented by drawing the atoms and their bonds using lines and dots. Web the cof2 lewis structure refers to the arrangement of atoms and electrons in carbon dioxide difluoride. Of2 co2 this problem has been solved! Include all.
Draw Lewis Structure
Web science chemistry chemistry questions and answers part a for cof2 draw an appropriate lewis structure (carbon is the central atom). Web now, let’s study the steps involved in drawing the lewis dot structure of carbonyl fluoride: Web chemistry questions and answers. Shared pairs of electrons are drawn as lines between atoms, while lone pairs.
Draw The Lewis Structure Of COF2. Include Lone Pairs.
Web drawing the lewis structure for cof 2. Search for the total number of valence electrons already available in a single carbonyl fluoride atom: Lewis structures expand/collapse global location 1.3: It is 24 as 4 are coming from the carbon atom, 6 are coming from the oxygen atom and 7 are coming from each of.
COF2 Lewis Structure How to Draw the Lewis Structure for COF2 YouTube
There are 2 steps to solve this one. You'll need to form a double bond between the carbon and oxygen to complete the octet on. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web drawing the lewis structure for cof 2. This problem has been solved! There.
Draw The Lewis Structure Of Cof2 . Include Lone Pairs Web science chemistry chemistry questions and answers part a for cof2 draw an appropriate lewis structure (carbon is the central atom). Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Lewis structures expand/collapse global location 1.3: Search for the total number of valence electrons already available in a single carbonyl fluoride atom: Web to properly draw the cof 2 lewis structure, follow these steps:
Web To Draw The Lewis Structure Of An Atom, Write The Symbol Of The Atom And Draw Dots Around It To Represent The Valence Electrons.
Web watch on contents how to draw lewis structure of cof2? Of2 co2 this problem has been solved! Web chemistry chemistry questions and answers draw the lewis structure of hcn.hcn. Web lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis structure.
There Are Three Violations To The Octet Rule:
Include all lone pair electrons in your structure. In the lewis structure for cof 2 there are a total of 24 valence electrons. Let’s draw and understand this lewis dot structure step by step. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.
Web In Lewis Electron Structures, We Encounter Bonding Pairs, Which Are Shared By Two Atoms, And Lone Pairs, Which Are Not Shared Between Atoms.
The lewis structure helps us understand the bonding and electron distribution in a molecule. If you write the cof2 over here, it will be double bond o and f. Web a second electron pair from each oxygen atom must be shared with the central carbon atom shown by the arrows above. For the cof2 structure use the periodic table to find the total number of valence electrons.
Include All Lone Pairs Of Electrons.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. In the cof 2 lewis structure carbon is least electron electronegative atom and goes in the center of the lewis structure. Web this problem has been solved! Sif4 secl2 cof2 (c is central) this problem has been solved!