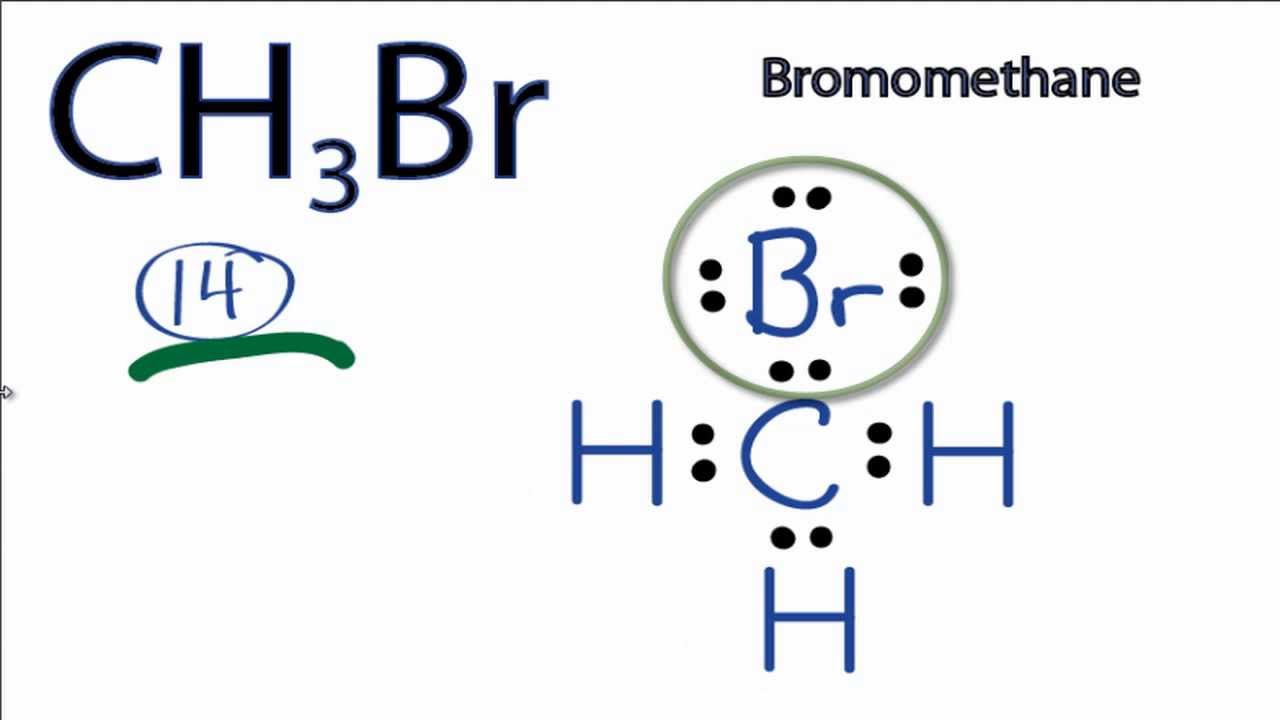
Draw The Lewis Structure Of Ch3Br
Draw The Lewis Structure Of Ch3Br - Web ch3br lewis structure has a carbon atom (c) at the center which is surrounded by three hydrogen atoms (h) and one bromine atom (br). Web this is the total number of electrons that must be used in the lewis structure. It is a chemical formula for bromomethane. Web in this section, we will draw the lewis structure of the ch 3 br molecule step by step: Web by using the following steps, you can easily draw the lewis structure of ch 3 br.
#1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. We will learn how to draw lewis structure of ch 3 br step by step in this tutorial. The second step is to calculate the ch3br hybridization, and the third step is to give. You can intuitively draw any lewis structure on your computer or mobile device. For the ch3br structure use the periodic table to find the total number of valence electrons for the ch3br. So the total number of valence electrons in ch3br is (4 + 3*1 + 7) = 14. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail.
How to draw CH3Br Lewis Structure? Science Education and Tutorials
For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. The type of hybridization around the br atom is sp3.3. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. O + 2 (f) = of 2..
draw the lewis structure of ch3br sectionalviewsengineeringdrawing
The bond length between the carbon and hydrogen atoms is 1.09 å.5. This widget gets the lewis structure of chemical compounds. It is a chemical formula for bromomethane. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. #1.
CH3Br Lewis Structure How to Draw the Lewis Structure for CH3Br
While selecting the center atom, always put the least. Web the lewis structure for ch3br is shown below: The type of hybridization around the br atom is sp3.3. First, the valence electrons are. For the ch3br structure use the periodic table to find the total number of valence electrons. O + 2 (f) = of.
[Solved] Draw the Lewis structure of bromoform (CH3Br). a. What is the
The central atom is carbon, which is bordered on four terminals with one bromine atom, three. There are 3 lone pairs on the bromine atom (br). The bond length between the carbon and hydrogen atoms is 1.09 å.5. There is a single bond between the carbon (c) & bromine (br) atom as well as between.
CHBr3 Molecular Geometry Science Education and Tutorials
Web the lewis structure for ch3br is shown below: Web ch3br lewis structure has a carbon atom (c) at the center which is surrounded by three hydrogen atoms (h) and one bromine atom (br). Web chemistry chemistry questions and answers draw the lewis structure of ch3br this problem has been solved! Draw the lewis structure.
How to draw CH3Br Lewis Structure? Science Education and Tutorials
Web check me out: Count the total number of valence electrons for all the atoms in the molecule. For the ch3br structure use the periodic table to find the total number of valence electrons for the ch3br. We will learn how to draw lewis structure of ch 3 br step by step in this tutorial..
CH3Br Lewis Structure (Bromomethane) YouTube
For ch3br, there are a total of 14 valence electrons. The type of hybridization around the br atom is sp3.3. Draw the lewis structure of ch3br draw the lewis structure of ch3br here’s the best way to solve it. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. Web see the big list of lewis structures. For the ch3br structure use the periodic table to find the total number of valence electrons. Web the lewis structure for ch3br is shown below: Shared pairs of electrons are drawn.
CH3Br (Bromomethane) Molecular Geometry, Bond Angles YouTube
#1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. For the ch3br structure use the periodic table to find the total number of valence electrons. Carbon atom is the.
Draw The Lewis Structure Of Ch3Br For the ch3br structure use the periodic table to find the total number of valence electrons. Here, the given molecule is ch3br. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When the question is first opened, a box with a.
#1 Draw A Rough Sketch Of The Structure #2 Next, Indicate Lone Pairs On The Atoms #3 Indicate Formal Charges On The Atoms, If Necessary Let’s Break Down Each Step In More Detail.
The second step is to calculate the ch3br hybridization, and the third step is to give. Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. In order to draw the lewis. We will learn how to draw lewis structure of ch 3 br step by step in this tutorial.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Draw the lewis structure of ch3br draw the lewis structure of ch3br here’s the best way to solve it. Here, the given molecule is ch3br. The bond length between the carbon and bromine. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.
Ch 3 Br Lewis Structure
For the ch3br molecule (c is the central atom), draw the correct lewis structure and select all polar covalent bonds. It has only one carbon atom. Web how to draw ch3br lewis structure? The bond length between the carbon and hydrogen atoms is 1.09 å.5.
Carbon Atom Is The Center Atom And Bromine Atom Has 3 Lone Pairs.
There is a single bond between the carbon (c) & bromine (br) atom as well as between the carbon (c) and hydrogen (h) atoms. O + 2 (f) = of 2. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Calculate the total number of valence electrons.