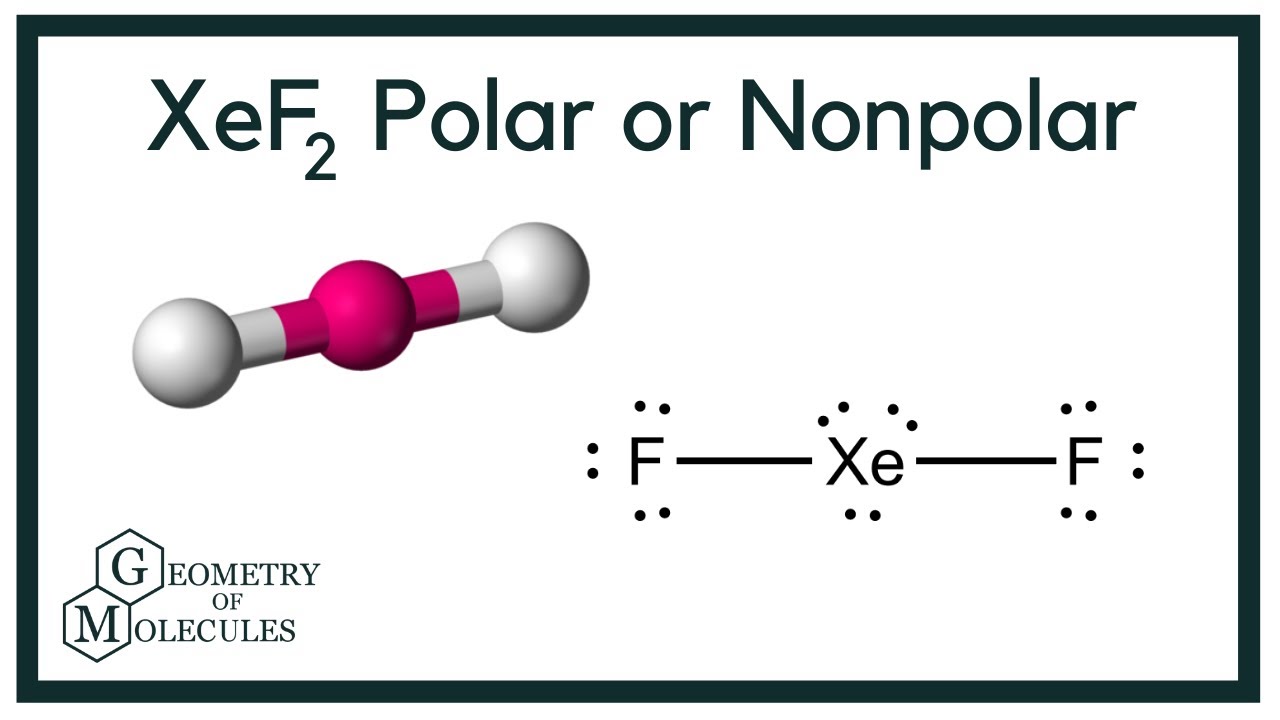Draw The Lewis Structure For The Xenon Difluoride Molecule
Draw The Lewis Structure For The Xenon Difluoride Molecule - Web chemistry chemistry questions and answers 6. Web in xef2 molecule, two fluorine atoms are arranged symmetrically on the outside with the central atom xenon in the middle. The lewis structure of xef6 shows that xenon is the central atom, surrounded by six fluorine atoms. Xef2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. Use vector addition to explain your answer.
Web chemistry chemistry questions and answers 6. See an example of a molecule that violates the octet rule (xef₂) and learn how to draw its lewis diagram in this video. Web drawing lewis structures for molecules with one central atom: Web xenon difluoride formula : Web in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). I also go over hybridization, shape and bond angle. Hence xenon difluoride is nonpolar as there is no polarity observed in the molecule.
Xenon difluoride, 99.5+, Thermo Scientific Chemicals Fisher Scientific
Hence xenon difluoride is nonpolar as there is no polarity observed in the molecule. If there are any atoms with a nonzero formal charge, be sure to write the formal charge next to the symbol. The xenon atom also holds 3 lone pairs of electrons. (1) find the number of valence electrons in the molecule..
Xenon Difluoride Molecular Structure Isolated on Black. 3d Illustration
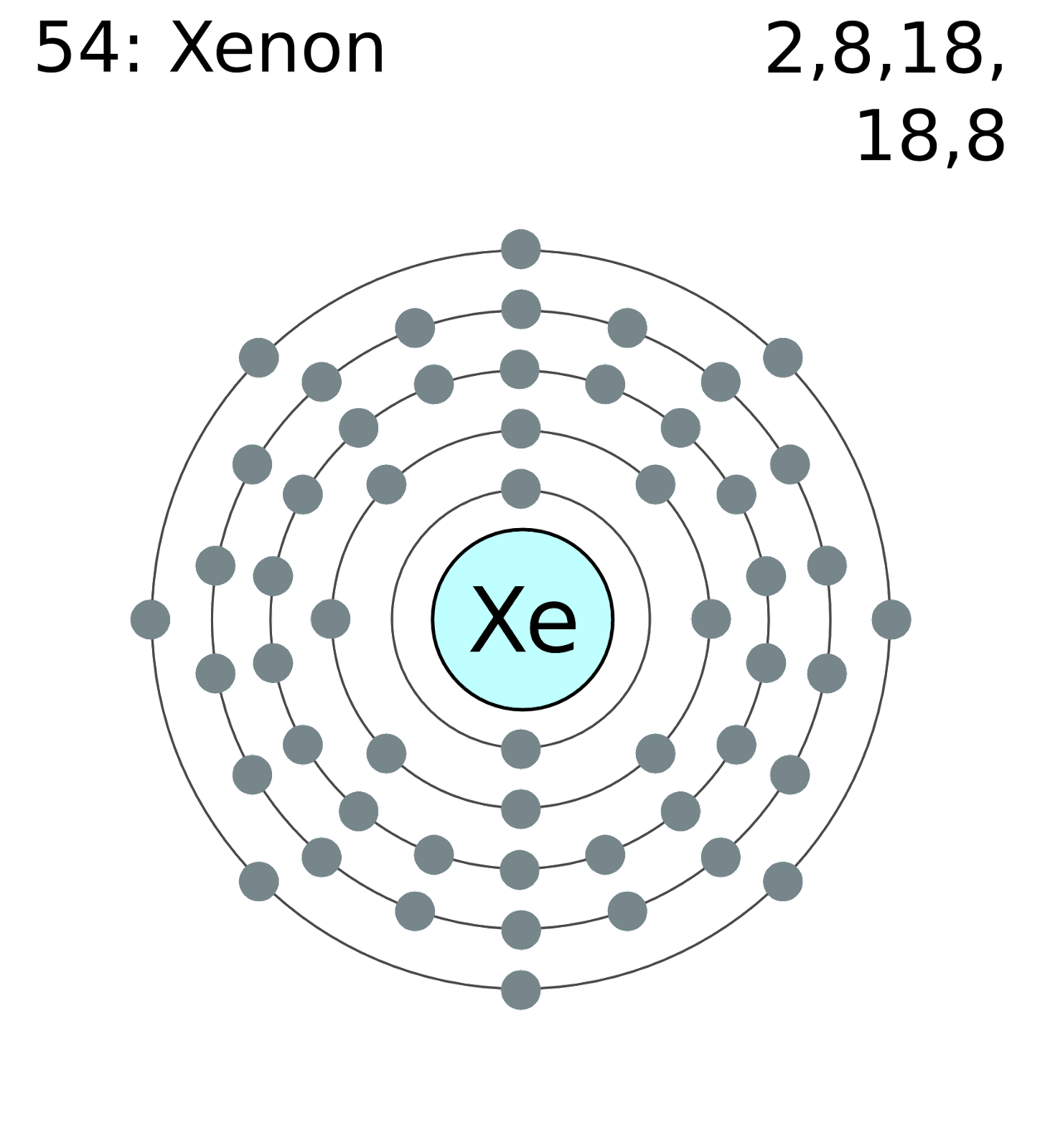
Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Web draw the lewis structure for the xenon difluoride (xef2) molecule. Now if we count the number of valence shell in xe we will find two electrons in the 5s orbital and six electrons in the 5p.
xenon difluoride Overview, Structure, Properties & Uses
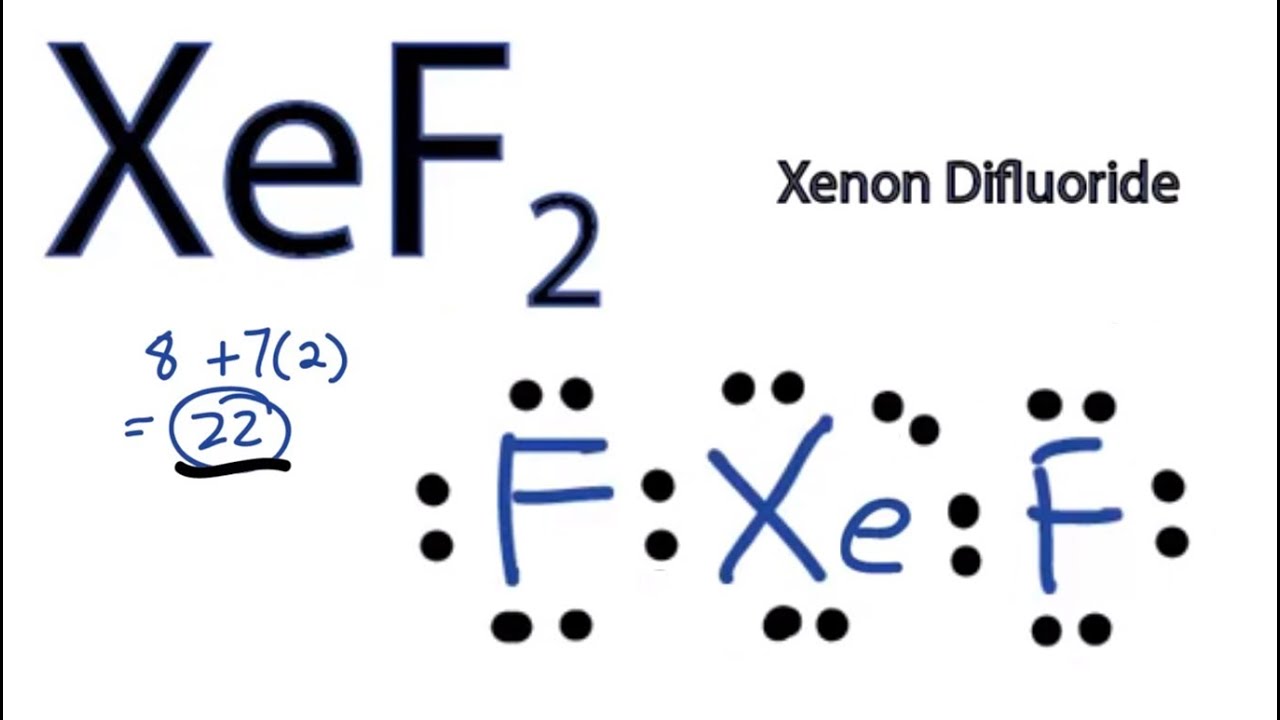
Web in xef2 molecule, two fluorine atoms are arranged symmetrically on the outside with the central atom xenon in the middle. Xef2 structure features two covalent bonds between one xenon atom and two fluorine atoms. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Web lewis structure of.
XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 YouTube
Web what is the lewis structure of xef2? If there are any atoms with a nonzero formal charge, be sure to write the formal charge next to the symbol. (b) (2 points) c the electronegativity of xe is 2.6. Let’s draw and understand this lewis dot structure step. Web in the hybridization of xenon difluoride,.
XeO2F2 Lewis Structure How to Draw The Lewis Structure for XeO2F2
Web in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom. This tutorial will help you deal with the lewis. However, in the excited state, its configuration will change to 5s.
The Lewis Structure Of Xef2 Understanding The Bonding Of Xenon
This tutorial will help you deal with the lewis. Use vector addition to explain your answer. Trigonal bipyramidal molecular geometry :. Web xeo2f2 is a chemical formula for xenon dioxy difluoride. If there are any atoms with a nonzero formal charge, be sure to write the formal charge next to the symbol. The xenon atom.
Hello Guys! Today we are going to look at the Lewis Structure of XeF2
The lewis structure of xef2 depicts the arrangement of atoms and valence electrons in a molecule of xenon difluoride. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web to draw the xef2 lewis structure, follow these steps: See an example of a.
XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Each fluorine atom forms a single bond with the. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web in xef2 molecule, two fluorine atoms are arranged symmetrically on the outside with the central atom xenon in the middle. Web chemistry chemistry questions.
Number of Lone Pairs and Bonding Pairs for XeF2 (Xenon difluoride
Hence xenon difluoride is nonpolar as there is no polarity observed in the molecule. Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom. (b) (2 points) c the electronegativity of xe is 2.6. (3) distribute the remaining electrons throughout the molecule, keeping in mind the duet and.
Xenon Difluoride Shape Draw Easy
Perspective drawing with bond angles: Web lewis structure of xef2 contains two single bonds between the xenon (xe) atom and each fluorine (f) atom. Xef2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The xenon.
Draw The Lewis Structure For The Xenon Difluoride Molecule Find the total valence electrons in xef2 molecule in order to find the total valence electrons in xef2 (xenon difluoride) molecule, first of all you should know the valence electrons present in xenon atom as well as fluorine atom. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web in the hybridization of xenon difluoride, xenon (xe) is the central atom. Web xenon difluoride formula : (1) find the number of valence electrons in the molecule.
Hence Xenon Difluoride Is Nonpolar As There Is No Polarity Observed In The Molecule.
It shows xenon (xe) as the central atom bonded to two fluorine (f) atoms. (a) (2 points) in the space provided below, draw a lewis structure for the molecule xenon difluoride, xef2. Web in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). Trigonal bipyramidal molecular geometry :.
Web To Draw The Xef2 Lewis Structure, Follow These Steps:
(4 points)what is the molecular shape of xenon difluoride? (2) draw single bonds between bonded atoms. Xef2 structure features two covalent bonds between one xenon atom and two fluorine atoms. Web the structure of xenon difluoride is illustrated below.
The Xenon Atom (Xe) Is At The Center And It Is Surrounded By 2 Fluorine Atoms (F).
Web chemistry chemistry questions and answers 6. Web in xef2 molecule, two fluorine atoms are arranged symmetrically on the outside with the central atom xenon in the middle. (3) distribute the remaining electrons throughout the molecule, keeping in mind the duet and octet rules. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons).
Web The Xef6 Lewis Structure Refers To The Arrangement Of Atoms And Electrons In A Molecule Of Xenon Hexafluoride.xenon Hexafluoride Is A Compound Composed Of One Xenon Atom Bonded To Six Fluorine Atoms.
There is no net dipole moment in the compound due to the arrangement of the valence electrons in symmetry. Find the total valence electrons in xef2 molecule in order to find the total valence electrons in xef2 (xenon difluoride) molecule, first of all you should know the valence electrons present in xenon atom as well as fluorine atom. (5 points) draw the lewis structure for xenon difluoride. Web xef2 lewis structure + molecular geometry chem101csub 3.77k subscribers subscribe 43 14k views 9 years ago chemistry learning made easy.