Draw The Lewis Structure For Sf4
Draw The Lewis Structure For Sf4 - Web and to draw the lewis structure of sf4, we first need to know the total number of valence. Web draw the lewis structure of sf4 showing all lone pairs. Sp3d, 1 od.sp3.0 o e. Rotate arrow right explore similar answers messages Here, the given molecule is sf4.
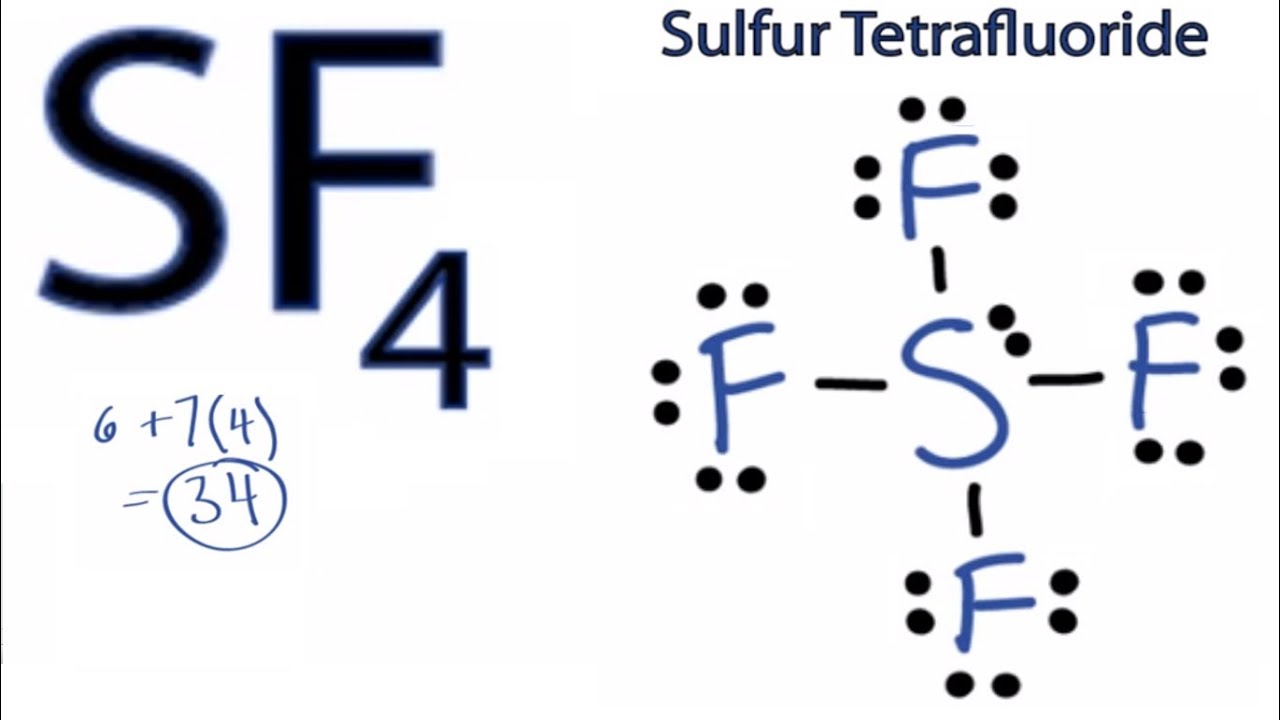
For the sf4 structure use the periodic table to find the total number of valence electrons. I also go over formal charge, hybridization, shape and bond angle. We'll put the fluorines around it, all four of them. Apart from this, there are 4 other fluorine atoms. What is the hybridization and formal charge on the sulfur? Fluorine is in group 17, so each fluorine atom contributes 7 valence electrons. Web by using the following steps, you can easily draw the lewis structure of sf 4:
How to draw SF4 Lewis Structure? Science Education and Tutorials
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. What is the hybridization and formal charge on the sulfur? This problem has been solved! Web watch on steps of drawing sf4 lewis structure step 1: Web science chemistry chemistry questions and answers draw the lewis structure for sf4.
SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
For sf4, sulfur has 6 valence electrons, and each fluorine atom has 7 valence electrons, so the total is 6 + 4 (7) = 34 electrons. Web and to draw the lewis structure of sf4, we first need to know the total number of valence. For the lewis structure for sf4 you should take formal.
SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
For the central sulfur atom: The number of single bonds =. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. We'll put the fluorines around it, all four of them. For the lewis structure for sf4 you should take formal charges into account to find the best lewis.
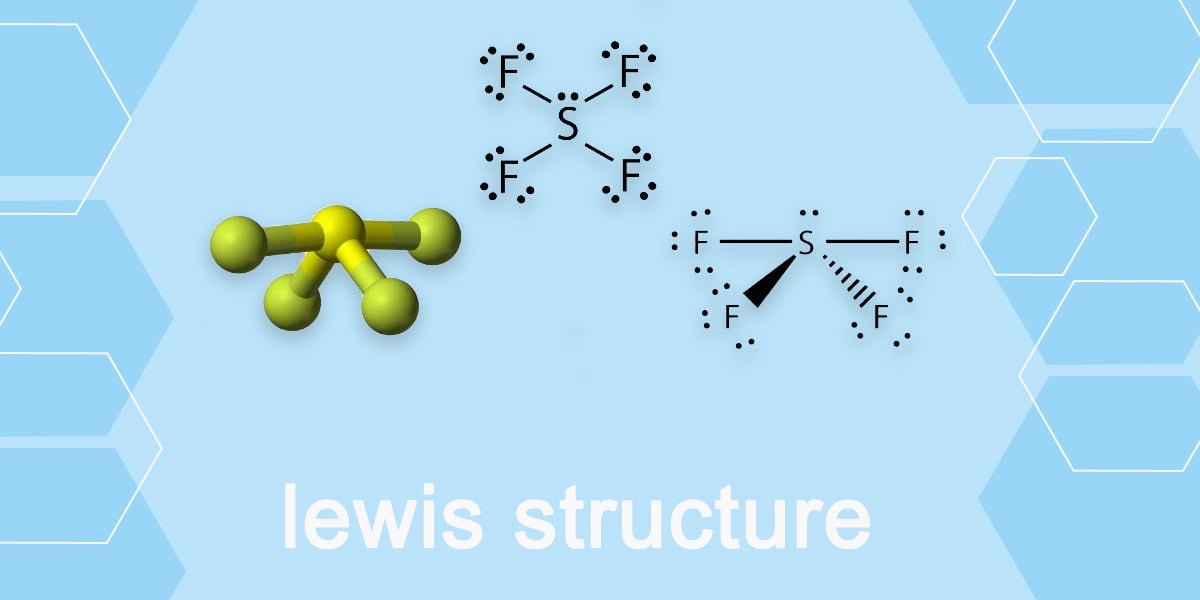
SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
#4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. 0 this problem has been solved! We'll put the s, the least electronegative, at the center. Web to draw the lewis structure for sf4, we start by determining the total number.
SF4 Lewis Dot structureHow to draw the Lewis structure for SF4. neet
Next, we put bonds between atoms. Sp3d, 1 od.sp3.0 o e. This problem has been solved! Sulfur is in group 16 of the periodic table, so it has 6 valence electrons. Web drawing lewis structures for molecules with one central atom: It is helpful if you: Web 5 steps to draw the lewis structure of.
SF4 Molecular Geometry, Lewis Structure, Bond Angles and Polarity
O trigonal bipyramidal what are the ideal bond angles of this geometry? In total, we have 6 + 4 (7) = 34 valence electrons. Determine the total number of valence electrons to begin, count the total number of valence electrons in the sf4 molecule. We'll put the fluorines around it, all four of them. Identify.
How to draw SF4 Lewis Structure? Science Education and Tutorials
Web sf 4 lewis structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. In order to draw the lewis. While selecting the atom, always put the least electronegative atom at the center. This problem has been solved! Here, the given molecule is sf4. You'll get a detailed.
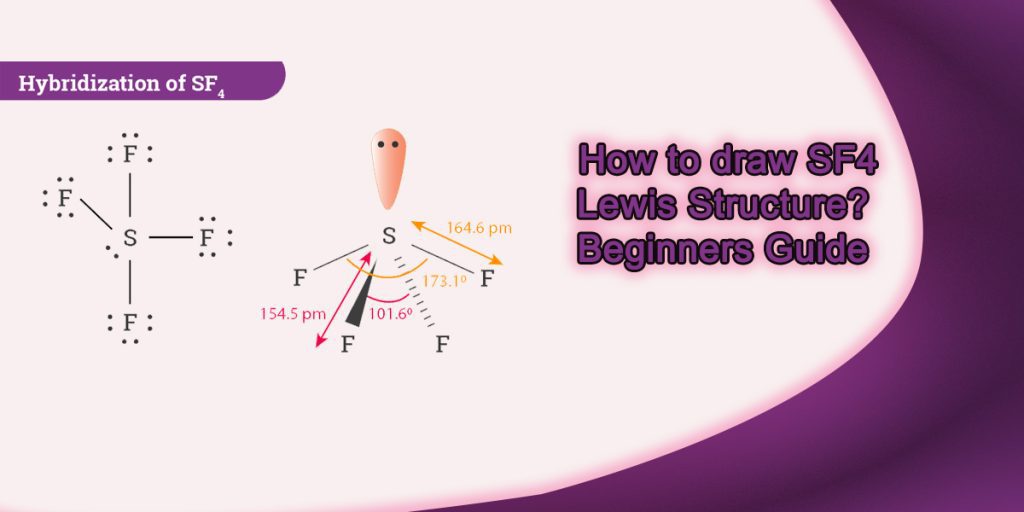
How to draw Sf4 Lewis Structure? Beginners Guide
For the sf4 structure use the periodic table to find the total number of valence electrons. This problem has been solved! Key points to consider when drawing the sf4 structure. We'll put the fluorines around it, all four of them. For the central sulfur atom: For a total of 34 valence electrons. Web science chemistry.
How to draw Sf4 Lewis Structure? Beginners Guide
Identify the molecular geometry of sfa. For the sf4 structure use the periodic table to find the total number of valence electrons. For the lewis structure for sf4 you should take formal charges into account to find the best lewis structure for the molecule. Web draw the lewis structure of sf4 showing all lone pairs..
SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Web drawing lewis structures for molecules with one central atom: Watch the video and see if you missed any steps or information. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Find.
Draw The Lewis Structure For Sf4 Determine the total number of valence electrons by adding up the valence electrons of all atoms in the molecule. Fluorine is in group 17, so each fluorine atom contributes 7 valence electrons. In total, we have 6 + 4 (7) = 34 valence electrons. Web 5 steps to draw the lewis structure of sf4 step #1: Web science chemistry chemistry questions and answers draw the lewis structure for sf4 in the window below and then answer the questions that follow.
Key Points To Consider When Drawing The Sf4 Structure.
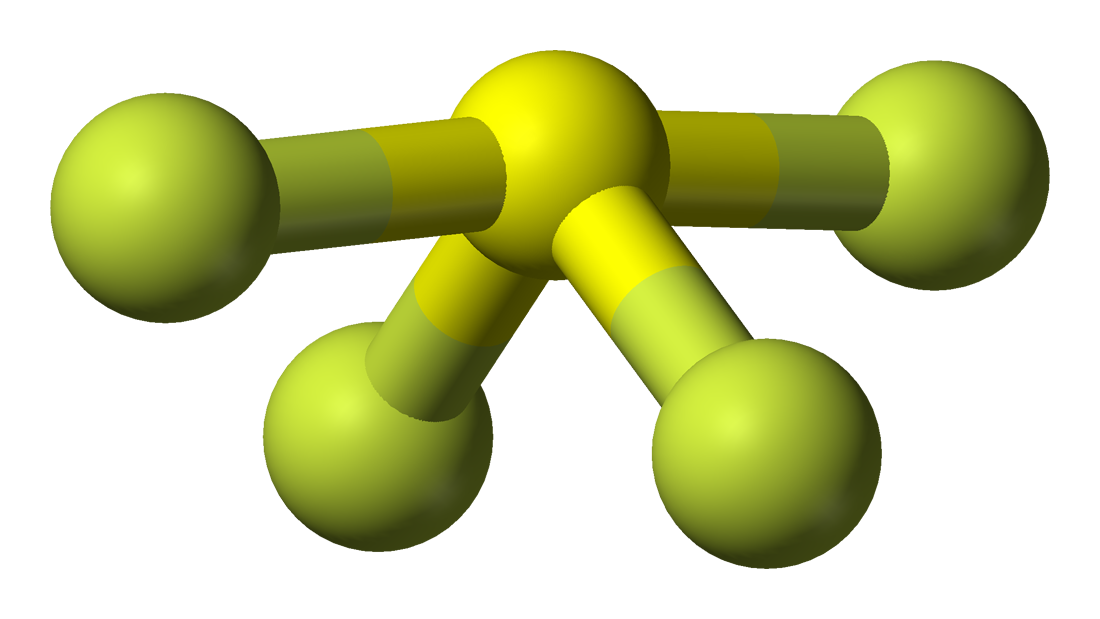
In this lewis structure of sf 4, center sulfur atom has made four single bonds with four fluorine atoms. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Identify the molecular geometry of sfa. Sp3d, 1 od.sp3.0 o e.
For The Lewis Structure For Sf4 You Should Take Formal Charges Into Account To Find The Best Lewis Structure For The Molecule.
Web drawing lewis structures for molecules with one central atom: On the periodic table, 6 valence electrons for sulfur, 7 for fluorine but we have 4 fluorines; This problem has been solved! Web draw the lewis dot structure for sf4 and determine the hybridization of the s atom and the f atoms.
Web Hello Guys!Today We Are Going To Look At The Lewis Structure Of Sf4 ( Xenon Tetrafluoride )The Sulphur Atom Has Six Valence Electrons In Its Outer Shell And.
Web there are a total of 34 valence electrons in the lewis structure for sf4. The central atom in this compound is sulfur. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. The central atom is sulfur, which is bordered on four terminals with fluorine atoms and one lone.
I Also Go Over Formal Charge, Hybridization, Shape And Bond Angle.
First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the atoms to form bonds and lone pairs. The number of double bonds =. Web to draw the lewis structure for sf4, we start by determining the total number of valence electrons. For the sf4 structure use the periodic table to find the total number of valence electrons.