Draw The Lewis Structure For Sf2
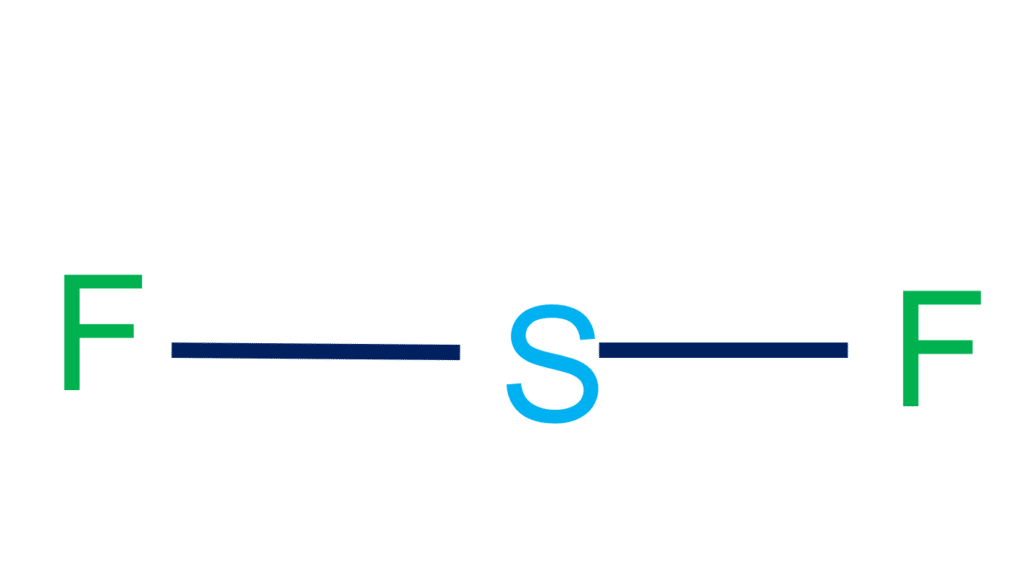
Draw The Lewis Structure For Sf2 - Include all the lone pairs. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Before we dive into drawing the lewis structure of sf2, it's crucial to gather some essential information about the molecule. The second step is to add valence electrons to the two fluorine atoms, and the final step is to combine the.
Web the lewis structure for sf 2 has 20 valence electrons available to work with. Web chemistry 360 14.9k subscribers subscribe share 2.2k views 3 years ago playlist:lewis structure (click here to learn more) sf2 lewis structure||lewis structure of sf2 (sulfur. Put the least electronegative atom in the center. #1 draw a rough skeleton structure first, determine the total number of valence electrons Here’s the best way to solve it. Web steps of drawing sf2 lewis structure step 1: It is important to look at what the lewis structure of sf2 is so that we can move ahead and look at other aspects of it.
Draw the lewis structure of sf2 showing all lone pairs torbucket
Sulfur has 6 valence electrons. Calculate the total number of valence electrons. Find the total valence electrons in sf2. Web the first step is to sketch the lewis structure of the sf2 molecule, to add valence electrons around the sulfur atom; Web chemistry questions and answers. Web draw the best lewis structure for sf2, including.
SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Web chemistry 360 14.9k subscribers subscribe share 2.2k views 3 years ago playlist:lewis structure (click here to learn more) sf2 lewis structure||lewis structure of sf2 (sulfur. Web here’s how you can easily draw the sf 2 lewis structure step by step: The lewis structure of sf2 is similar to the sbr2 and scl2. Web steps.
Draw the lewis structure of sf2 showing all lone pairs punchstart
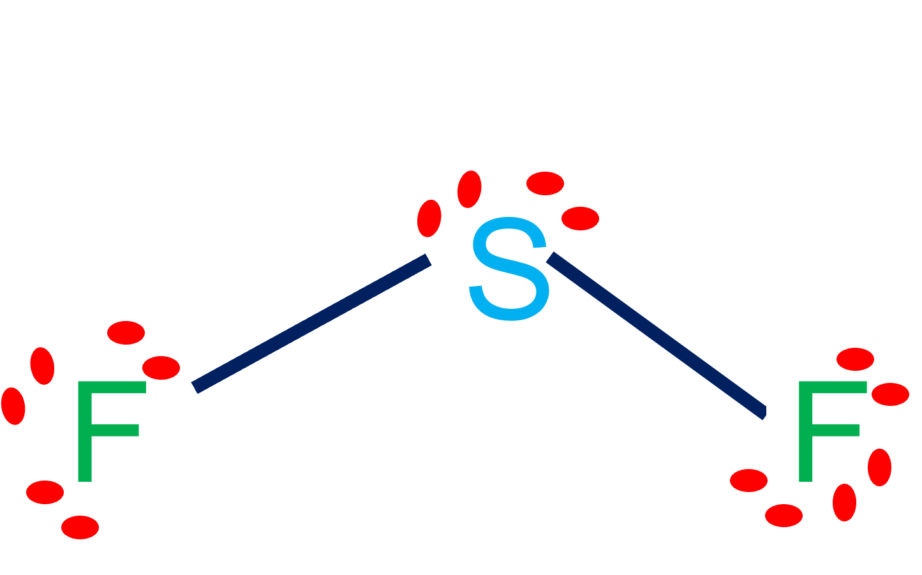
#1 draw a rough skeleton structure first, determine the total number of valence electrons Sf2 consists of one sulfur (s) atom and two fluorine (f) atoms. For the central sulfur atom: Draw the lewis structure of sf2, showing all lone pairs. What is the approximate bond angle in sf2? Valence electrons of sulfur are 6.
So far, we’ve used 20 of the SF2 Lewis structure’s total 20 outermost
Web how to draw lewis structure of sf2 step 1: Web draw the best lewis structure for sf2, including all nonbonding electrons and all nonzero formal charges if applicable. For the central sulfur atom: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure of.
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
In sf2, the sulfur atom (s) is less electronegative than fluorine (f), so it will be. Sf2 consists of one sulfur (s) atom and two fluorine (f) atoms. To determine the total number of valence electrons in sf2, we need to. Web lewis structure is nothing but an arrangement of valence electrons between different atoms..
How to draw SF2 Lewis Structure? Science Education and Tutorials
Draw the lewis structure of sf2. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a closer look at each step mentioned above. Web chemistry chemistry questions and answers draw a lewis structure for sf2 that has minimized formal.
SF2 Lewis Structure How to Draw the Lewis Structure for SF2 YouTube
Select a tool to begin drawing < 0506 macbook pro 36 3む procasemand this problem has been solved! The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. The lewis structure of sf2 is similar to the sbr2 and scl2. Include all lone pairs.
SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Calculate the total number of valence electrons. Web science chemistry chemistry questions and answers draw the lewis structure of sf2. Draw the lewis structure of sf2, showing all lone pairs. Sulfur has 6 valence electrons. #1 draw a rough skeleton structure first, determine the total number of valence electrons The second step is to add.
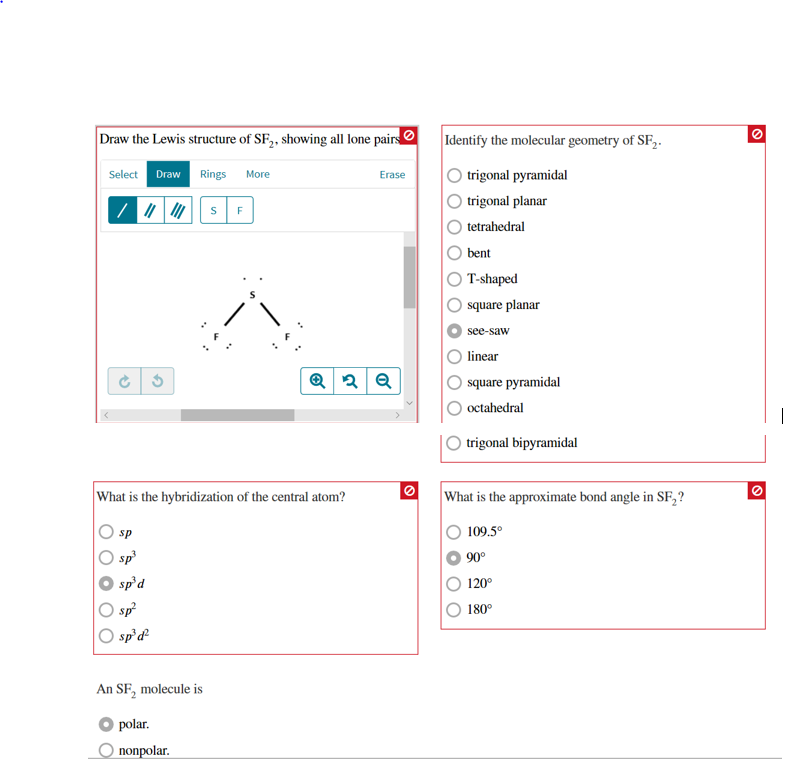
Draw the Lewis structure of SF2, showing all lone pairs. Identify the
Web how to draw lewis structure of sf2 step 1: Web chemistry questions and answers. Find the total valence electrons in sf2 molecule in order to find the total valence electrons in sf2 (sulfur difluoride) molecule, first of all you should know the valence electrons present in sulfur atom as well as fluorine atom. The.
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
Identify the molecular geometry of sf2. Sf2 consists of one sulfur (s) atom and two fluorine (f) atoms. Web draw lewis structure for sf2. Draw the molecule by placing atoms on the grid and connecting them with bonds. First, we will have to calculate the total number of valence electrons in this compound. Web chemistry.
Draw The Lewis Structure For Sf2 Web chemistry 360 14.9k subscribers subscribe share 2.2k views 3 years ago playlist:lewis structure (click here to learn more) sf2 lewis structure||lewis structure of sf2 (sulfur. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a closer look at each step mentioned above. Sf2 consists of one sulfur (s) atom and two fluorine (f) atoms. Web lewis structure is nothing but an arrangement of valence electrons between different atoms. For the central sulfur atom:
#1 Draw A Rough Skeleton Structure #2 Mention Lone Pairs On The Atoms #3 If Needed, Mention Formal Charges On The Atoms Now, Let’s Take A Closer Look At Each Step Mentioned Above.
This problem has been solved! Identify the molecular geometry of sf2. Let's do the sf2 lewis structure. In sf2, the sulfur atom (s) is less electronegative than fluorine (f), so it will be.
Here, The Given Molecule Is Sf2 (Sulfur Difluoride).
What is the approximate bond angle in sf2? The number of lone pairs = the number of single bonds = the number of double bonds = 2. First, we will have to calculate the total number of valence electrons in this compound. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom.
Web Draw The Best Lewis Structure For Sf2, Including All Nonbonding Electrons And All Nonzero Formal Charges If Applicable.
Include all the lone pairs. Web the main objective of the question is to draw the lewis structure for sf a 2 along with its molecular geom. Web steps of drawing sf2 lewis structure step 1: Draw a lewis structure for sf2 that obeys the octet rule if possible and answer the following questions based on your drawing.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Here sulphur (atomic number = 16 and electronic configuration = 2,8,6) has 6 valence electrons. Web draw lewis structure for sf2. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web how to draw lewis structure of sf2 step 1: