Draw The Lewis Structure For No+
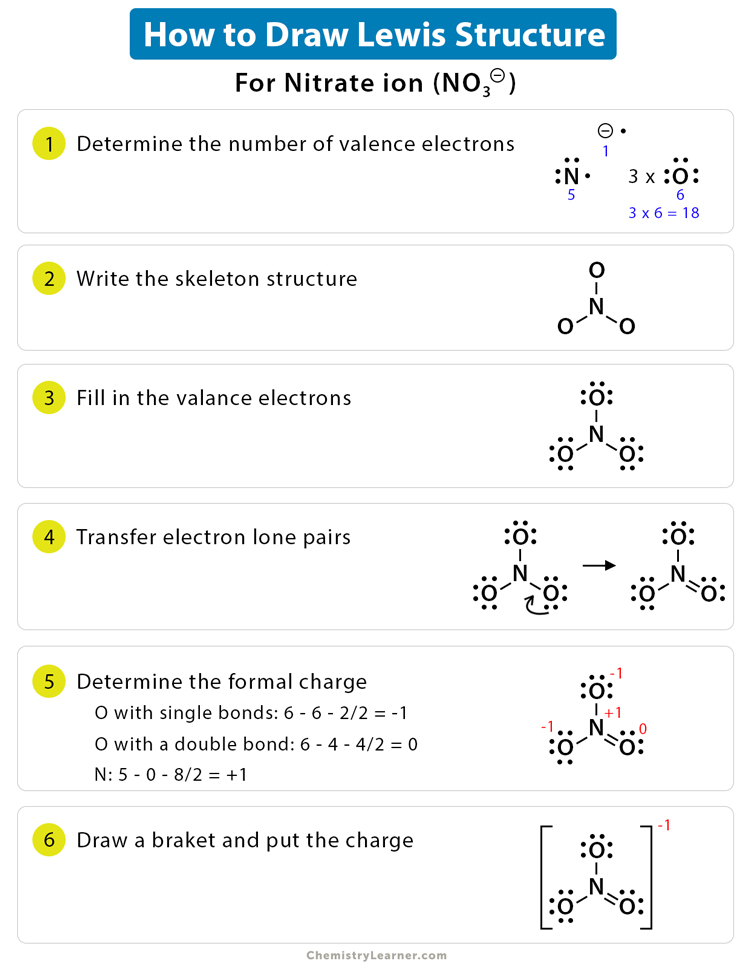
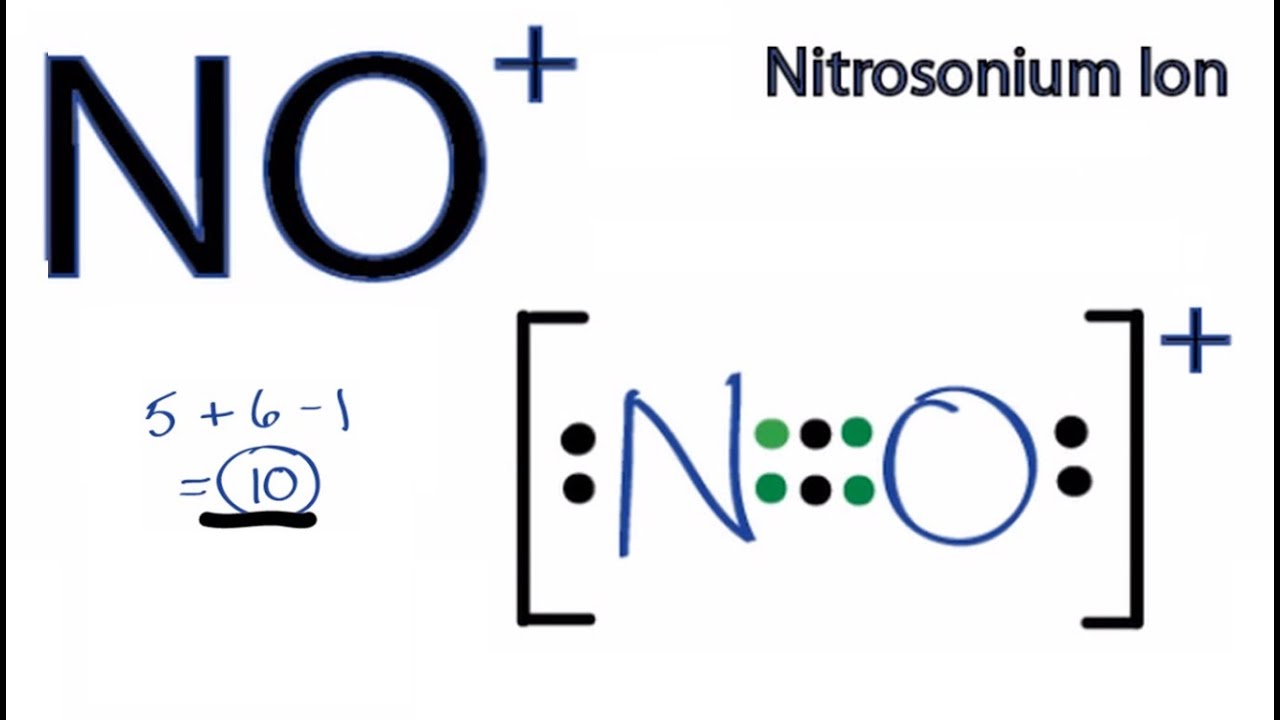
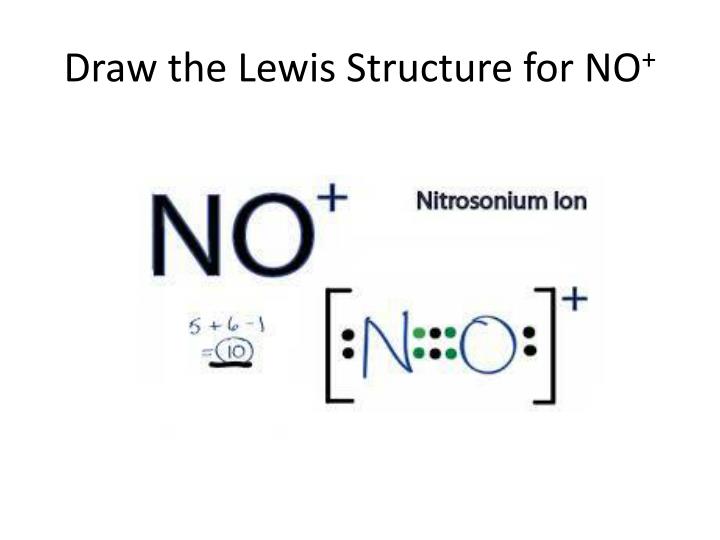
Draw The Lewis Structure For No+ - Be sure to put brackets and a positive sign around the no + lewis structure to show that it is an ion. View available hints) this problem has been solved! Web draw the lewis structure for no+. There are a total of 10 valence electrons in no +. Determine the total number of valence (outer shell) electrons.
For the no+ structure use the periodic table to find the total number of valence electrons for the. The plus sign up here actually means we're going to take away one of the valence electrons, for a total of 10 valence electrons for no+. Web 22k views 1 year ago. They should be properly referred to as major or minor resonance contributors/forms. Web steps of vsepr rule to draw lewis structure of no find total number of electrons of the valance shells of nitrogen atom and oxygen atom total electrons pairs center atom selection put lone pairs on atoms check the stability and minimize charges on atoms by converting lone pairs to bonds until most. | channels for pearson+ general chemistry 11. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom.
Lewis structure of NO+ (Nitrosonium ion) YouTube
Determine the total number of valence (outer shell) electrons. Web draw the lewis structure for no+. First, no+ lewis strucutre is usually drawn with a triple bond, and the + charge is put with a bracket to both ions. The “best” lewis structure is one that has the fewest formal charges — the top structure..
NO Lewis Structure How To Draw The Lewis Structure For NO(Nitric
I looked up a textbook to check the rules of determining which resonance structure contributes more. The sum of the valence electrons is. Be sure to put brackets and a positive sign around the no + lewis structure to show that it is an ion. There are a total of 10 valence electrons in no.
Draw Lewis Structure
They should be properly referred to as major or minor resonance contributors/forms. There are a total of 10 valence electrons in no +. I looked up a textbook to check the rules of determining which resonance structure contributes more. Web drawing the lewis structure for no + viewing notes: Web things to remember 1. You'll.
Lewis Dot Structure Definition, Examples, and Drawing
Determine the lewis dot structure for the following ion:scl 42+. I also go over hybridization, shape and bond angles. Web drawing lewis structures for molecules with one central atom: Count the total valence electrons present in no+ no + consists of two distinct elements, i.e., nitrogen and oxygen. There are a total of 10 valence.
Organic Chemistry How To Draw Lewis Structures YouTube
Carbon (c) will always be the central atom and hydrogen (h) will never be the central atom 2. Bonding & molecular structure lewis dot structures: I looked up a textbook to check the rules of determining which resonance structure contributes more. Nitrogen (n) is present in group v a (or 15) of the periodic table.
Beginner's Guide to Drawing Lewis Structures YouTube
I also go over hybridization, shape and bond angles. This problem has been solved! The plus sign up here actually means we're going to take away one of the valence electrons, for a total of 10 valence electrons for no+. Count the total valence electrons present in no+ no + consists of two distinct elements,.
Draw the Lewis structure for NO+ Is the nitrogenoxygen bond in NO+
Web things to remember 1. Web steps of vsepr rule to draw lewis structure of no find total number of electrons of the valance shells of nitrogen atom and oxygen atom total electrons pairs center atom selection put lone pairs on atoms check the stability and minimize charges on atoms by converting lone pairs to.
3 Ways to Draw Lewis Dot Structures wikiHow
Draw the lewis dot structure for ammonium chloride, nh 4 cl. The sum of the valence electrons is. Web 22k views 1 year ago. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. The plus sign up here actually means we're going to take away.
NO+ Lewis Structure How to Draw the Lewis Structure for NO+ YouTube
There are a total of 10 valence electrons in no +. They should be properly referred to as major or minor resonance contributors/forms. Web lewis structure for no+. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web steps for drawing the lewis.
PPT Molecular Structure & Intermolecular Forces PowerPoint
For the no+ structure use the periodic table to find the total number of valence electrons for the. Web chemistry chemistry questions and answers draw lewis structure (s) for the nitronium ion ( no2+ ). View available hints) this problem has been solved! I looked up a textbook to check the rules of determining which.
Draw The Lewis Structure For No+ With no + be sure to remove a valence electron from your total because of the positive sign. There are a total of 10 valence electrons in no +. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. First, no+ lewis strucutre is usually drawn with a triple bond, and the + charge is put with a bracket to both ions. View available hints) this problem has been solved!
This Problem Has Been Solved!
This problem has been solved! Web chemistry chemistry questions and answers draw the lewis structure for no. I looked up a textbook to check the rules of determining which resonance structure contributes more. Web there is no such thing as a better or a worse lewis structure.
The Sum Of The Valence Electrons Is.
View available hints) this problem has been solved! I need to know two things. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the molecule by placing atoms on the grid and connecting them with bonds.
For The No+ Structure Use The Periodic Table To Find The Total Number Of Valence Electrons For.
Draw the lewis dot structure for ammonium chloride, nh 4 cl. (valence electrons are the number of electrons present in the outermost shell of an atom). Web i quickly take you through how to draw the lewis structure of no+ (nitrosonium ion). First, no+ lewis strucutre is usually drawn with a triple bond, and the + charge is put with a bracket to both ions.
Be Sure To Put Brackets And A Positive Sign Around The No + Lewis Structure To Show That It Is An Ion.
Web steps of vsepr rule to draw lewis structure of no find total number of electrons of the valance shells of nitrogen atom and oxygen atom total electrons pairs center atom selection put lone pairs on atoms check the stability and minimize charges on atoms by converting lone pairs to bonds until most. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw the lewis structure for no+.