Draw The Lewis Structure For Asf3
Draw The Lewis Structure For Asf3 - Draw the lewis structure for bro2−. The lewis structure for asf 3 is similar to ascl 3 structure. Calculate the total number of valence electrons. All other atoms are bonded directly to the central atom. Draw the lewis structure for brf3.
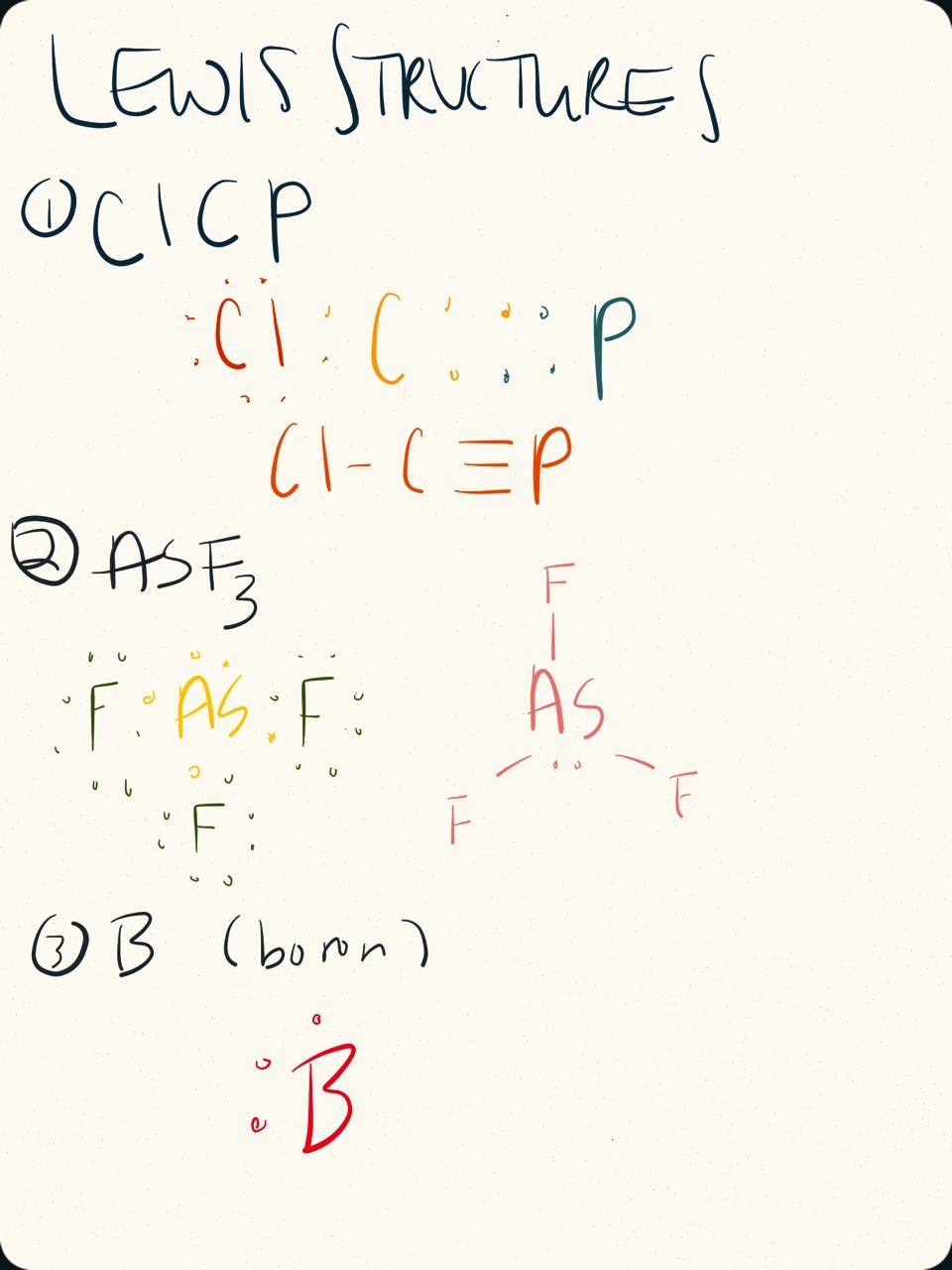
Calculate the total number of valence electrons. Draw the lewis structure for ch3+. Web 6 steps to draw the lewis structure of asf3 step #1: All other atoms are bonded directly to the central atom. The arsenic atom goes in the center of the lewis structure since it is the least. Web in its most stable state, the central arsenic atom forms three covalent bonds with the surrounding fluorine atoms. Web steps of drawing asf3 lewis structure step 1:
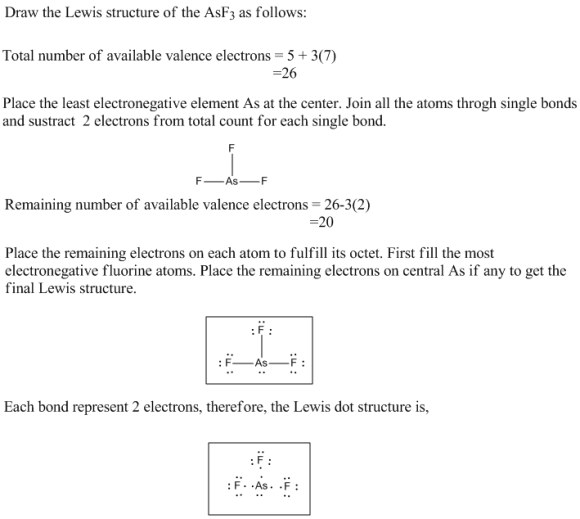
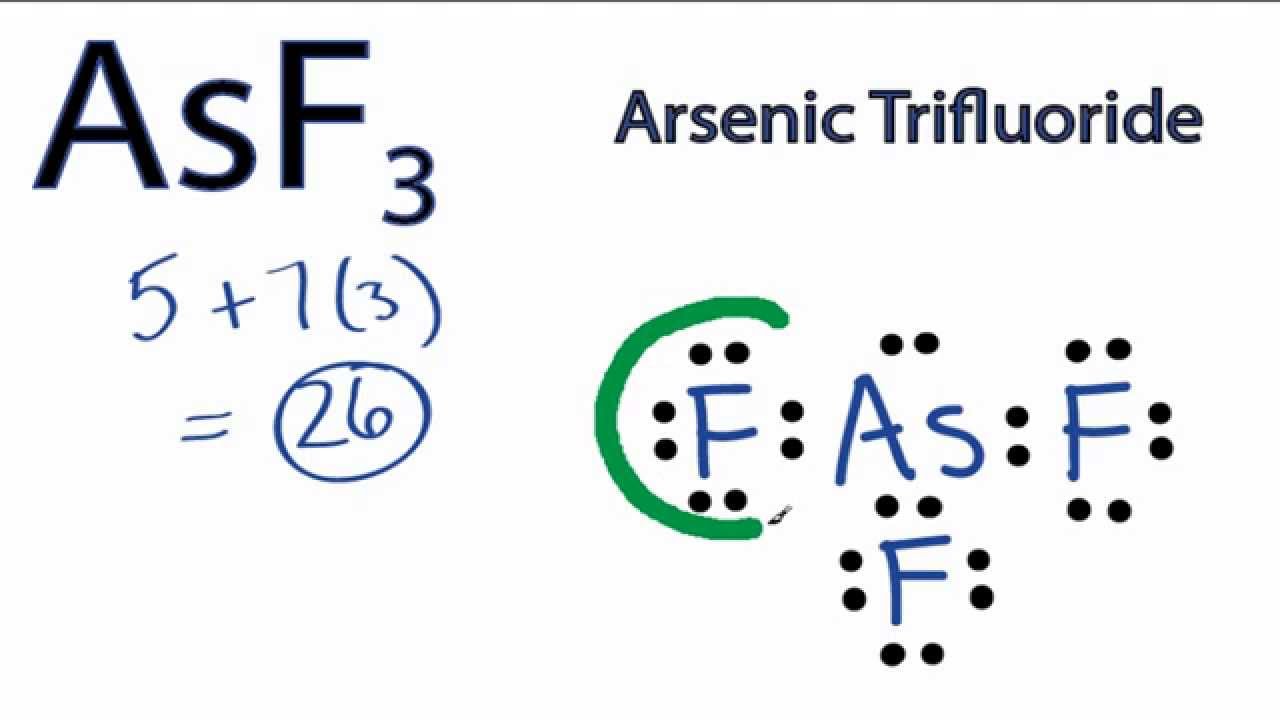
Draw the Lewis structure for AsF3 Draw the Lewis dot structure. To
The hybridization of the central arsenic atom in asf3 is sp3. Web steps of drawing asf3 lewis structure step 1: Web in its most stable state, the central arsenic atom forms three covalent bonds with the surrounding fluorine atoms. The lewis structure for asf 3 is similar to ascl 3 structure. The following procedure can.
AsF3 (Arsenic trifluoride) Molecular Geometry, Bond Angles YouTube
Web how to draw asf 3 lewis structure? Draw the lewis structure for asf3 draw the lewis dot structure. Find more chemistry widgets in wolfram|alpha. The following procedure can be used to draw lewis structure for simple molecules. Draw lewis structures for covalent compounds. [10%] determine the steric numbers of these compounds. _____ (b) what.
How to draw AsF3 Lewis Structure? 4
All other atoms are bonded directly to the central atom. 2.draw the lewis structure for ch3+. Draw the molecule by placing atoms on the grid and connecting them with bonds. [10%] determine the steric numbers of these compounds. Web drawing the lewis structure for asf 3 (arsenic trihydride) viewing notes: It is basically a structural.
MATHS HELP
[20%] (b) identify which of the compounds in (a) is a lewis acid, a lewis base or a radical. Include all lone pairs of electrons. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. _____ (b) what is the molecular.
Draw the Lewis structure for AsF3 YouTube
Find more chemistry widgets in wolfram|alpha. Web to determine its lewis structure we first check the total number of valence electrons for this molecule and then go through the arrangement of atoms, bond formation and the overall structure. This widget gets the lewis structure of chemical compounds. Web drawing the lewis structure for asf 3.
SOLVED Draw the Lewis structure for each of the following molecules or
Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Asf2, asf3, asf4, asf5 and [asf6] : Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trifluoride (asf3). Web (a) draw lewis structures for the following chemical species: Draw the molecule.
AsF3 Lewis Structure How to Draw the Lewis Structure for Arsenic
Asf2, asf3, asf4, asf5 and [asf6] : The arsenic atom goes in the center of the lewis structure since it is the least. The lewis structure for asf 3 is similar to ascl 3 structure. Calculate the total number of valence electrons. Web to determine its lewis structure we first check the total number of.
AsF3 Lewis Structure (Arsenic Trifluoride) Lewis, Molecules, Electrons
[20%] (b) identify which of the compounds in (a) is a lewis acid, a lewis base or a radical. _____ (b) what is the molecular geometry? Here, the given molecule is asf3. Web science chemistry chemistry questions and answers complete the following: How to draw the lewis structure for arsenic trifluoride. Web 6 steps to.
How to draw AsF3 Lewis Structure? 3
Find more chemistry widgets in wolfram|alpha. This widget gets the lewis structure of chemical compounds. Since they are in the same group on the periodic table they each have the same number of electrons their structures are similar. Web the lewis structure of asf3 contains three single bonds, with arsenic in the center, and three.
Asf3 Lewis Structure
Since they are in the same group on the periodic table they each have the same number of electrons their structures are similar. Web steps of drawing asf3 lewis structure step 1: Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are.
Draw The Lewis Structure For Asf3 Draw the lewis structure for brf3. Calculate the total number of valence electrons. Each fluorine atom has 3 lone pairs and arsenic atom has one lone pair. Web how to draw asf 3 lewis structure? [10%] determine the steric numbers of these compounds.
The Lewis Structure For Asf 3 Is Similar To Ascl 3 Structure.
#1 first draw a rough sketch first, determine the total number of valence electrons periodic table In order to draw the lewis. How to draw the lewis structure for arsenic trifluoride. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected.
Web Drawing Lewis Structures For Molecules With One Central Atom:
Draw the lewis structure for xef2. Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trifluoride (asf3). Draw the lewis structure for ch3+. All other atoms are bonded directly to the central atom.
There Are Three Lone Pairs On Each Fluorine Atom, And One Lone Pair On The Arsenic Atom.
[10%] determine the steric numbers of these compounds. While selecting the atom, always put the least electronegative atom at the center. Breaking the octet rule ; Asf2, asf3, asf4, asf5 and [asf6] :
Web (A) Draw Lewis Structures For The Following Chemical Species:
To know the process of drawing a lewis structure, first you have to know what is lewis structure. Web in its most stable state, the central arsenic atom forms three covalent bonds with the surrounding fluorine atoms. _____ (b) what is the molecular geometry? Each fluorine atom has 3 lone pairs and arsenic atom has one lone pair.