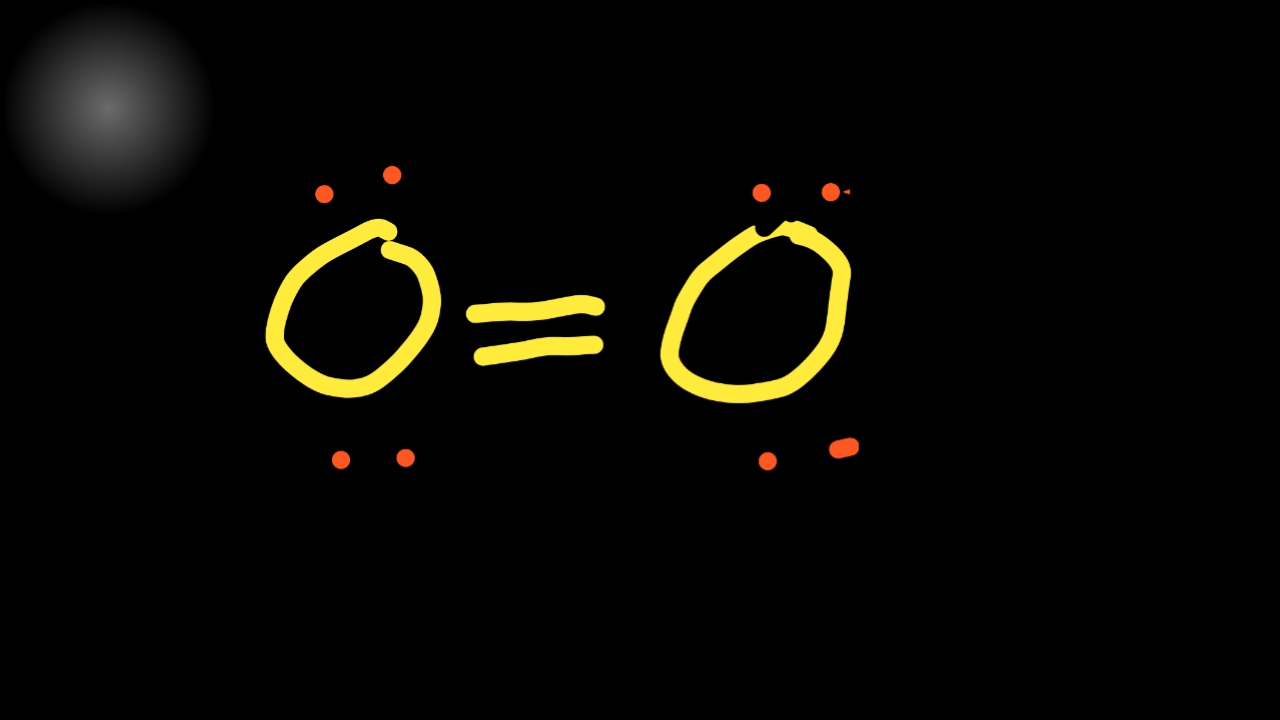Draw The Lewis Structure For A Oxygen O2 Molecule
Draw The Lewis Structure For A Oxygen O2 Molecule - In this case, there is no central atom, so we distribute the electrons around both atoms. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. In order to make sure the outer shell of the oxygen. There are many things to learn when we draw the o 2 lewis structure. The o 2 molecule is diatomic, meaning that two atoms of the same element are connected in.
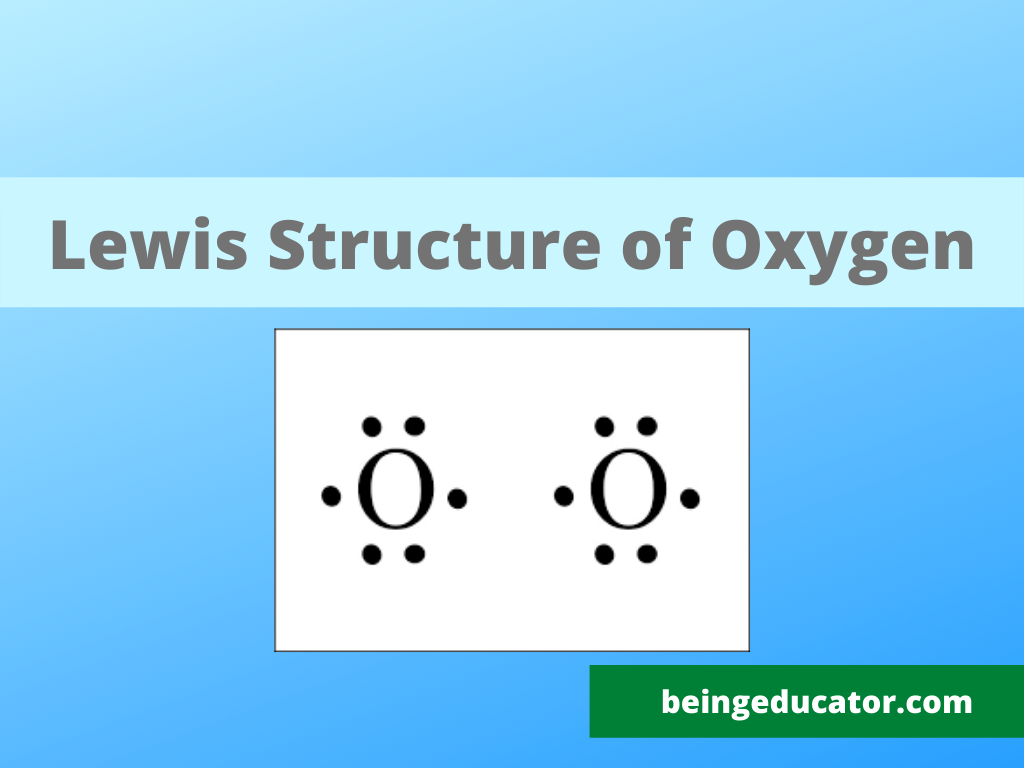
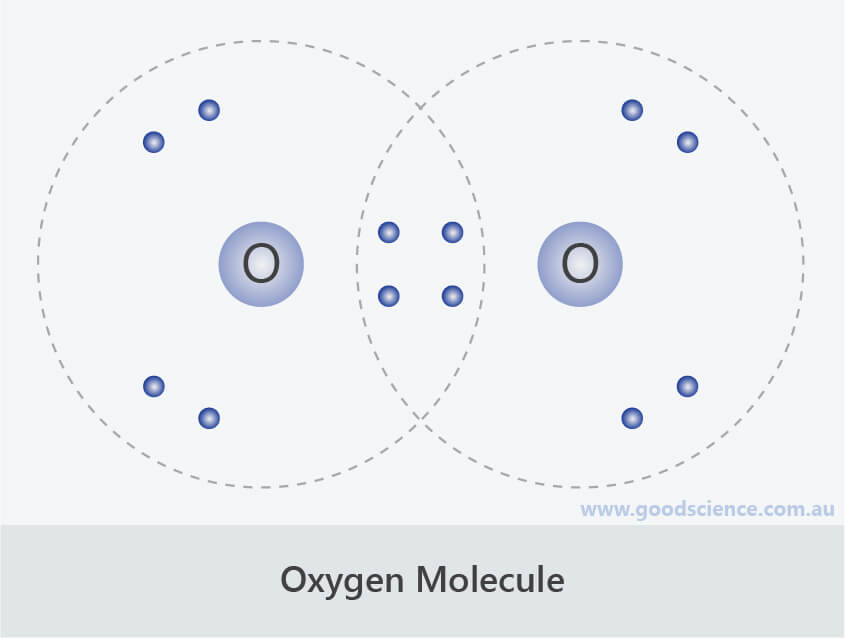
These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. Here we will take co 2 molecule as an example to explain the procedure step by step:. Web in the lewis structure of o2 structure there are a total of 12 valence electrons. Atoms can form more than one bond. Web lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. Oxygen gas watch on steps of drawing o2 lewis structure step 1:
O2 oxygen molecule Royalty Free Vector Image VectorStock
Web in the lewis structure of o2 structure there are a total of 12 valence electrons. Determine the total number of valence electrons in the molecule or ion. O 2 ( oxygen) has two oxygen atoms. Web the lewis structure of xef 2 shows two bonding pairs and three lone pairs of electrons around the.
O2 2 Lewis Structure How to Draw the Lewis Structure for O2 2 YouTube
Here we will take co 2 molecule as an example to explain the procedure step by step:. Be sure to draw all bonds and lone pairs. Note that each atom must contribute one electron to the bond. Web the lewis structure of xef 2 shows two bonding pairs and three lone pairs of electrons around.
O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Steps for writing lewis structures. Web o 2 lewis structure. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. How to draw the lewis structure for oxygen gas (diatomic oxygen) watch on. Where six are arranged, around each oxygen atom in a way that one side has four valence.
【2 Step】O2 Lewis StructureLewis Dot Structure for Oxygen(O,O2)Lewis
The o 2 lewis structure has a double bond between two oxygen atoms. O 2 ( oxygen) has two oxygen atoms. Steps for writing lewis structures. Two electrons remain, and this lone pair is placed on the xe atom: Web we draw lewis structures to predict: In order to make sure the outer shell of.
O2 Lewis Structure
Two electrons remain, and this lone pair is placed on the xe atom: In order to make sure the outer shell of the oxygen. The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Web the lewis structure of xef 2 shows two bonding pairs and three lone pairs of.
How to Draw the Lewis Dot Structure for O2 Oxygen gas YouTube
Web using lewis structures, we can represent this as follows: In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. In order to make sure the outer shell of the oxygen..
Ionic and Covalent Compounds Good Science
Oxygen gas watch on steps of drawing o2 lewis structure step 1: Web 6 steps to draw the lewis structure of o2 step #1: Oxygen is in group 16 of the periodic table, so it has 6 valence electrons. O 2 ( oxygen) has two oxygen atoms. Web lewis structures, introduction, formal charge, molecular geometry,.
O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
I also go over shape and bond angle. Two electrons remain, and this lone pair is placed on the xe atom: Oxygen gas watch on steps of drawing o2 lewis structure step 1: The first step is to sketch the lewis structure of the o2 molecule, to add valence electrons around the two oxygen atoms,.
Oxygen, O2, molecule model and chemical formula. Also dioxygen
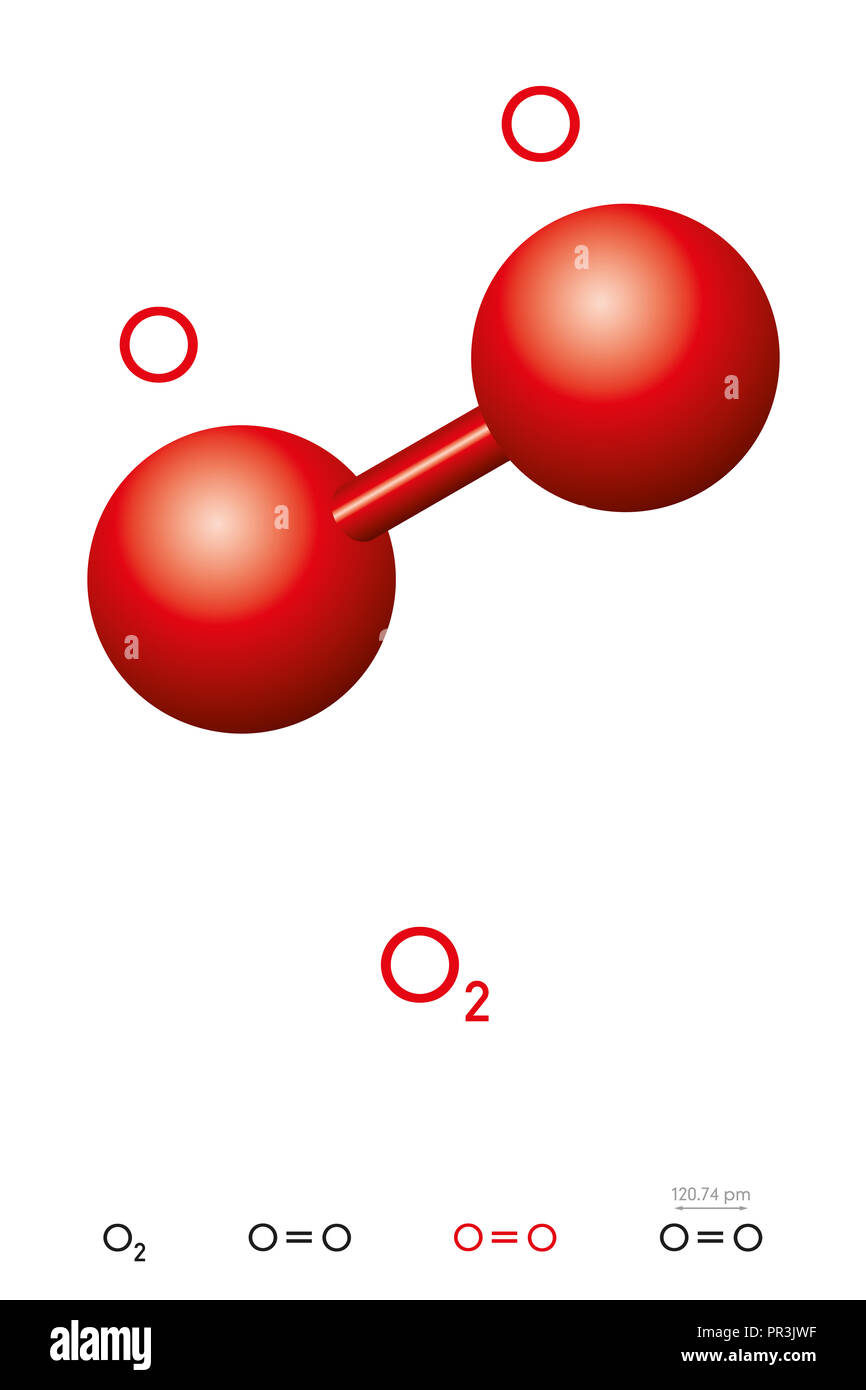
The o 2 lewis structure has a double bond between two oxygen atoms. In this case, there is no central atom, so we distribute the electrons around both atoms. 1st attempt part 1 (1 point) draw the lewis structure for o2. Determine the total number of valence electrons in the molecule or ion. The o.
How to Draw O2 Lewis Structure? 3
We place three lone pairs of electrons around each f atom, accounting for 36 electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. Web i quickly take you through how to draw the lewis structure of o2 (oxygen). Total number of valence electrons: In.
Draw The Lewis Structure For A Oxygen O2 Molecule O 2 ( oxygen) has two oxygen atoms. Web we draw lewis structures to predict: Oxygen is in group 16 of the periodic table, so it has 6 valence electrons. Web to draw the lewis structure of o2, we first need to determine the number of valence electrons for each oxygen atom. The o 2 molecule is diatomic, meaning that two atoms of the same element are connected in.
Two Fluorine Atoms Can Form A Molecule Of F 2 In The Same Fashion.
The o 2 molecule is diatomic, meaning that two atoms of the same element are connected in. Here we will take co 2 molecule as an example to explain the procedure step by step:. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons. The first step is to sketch the lewis structure of the o2 molecule, to add valence electrons around the two oxygen atoms, and the final step is to combine the two oxygen diatomic atoms to get the o2 lewis structure.
Oxygen Gas Watch On Steps Of Drawing O2 Lewis Structure Step 1:
4 (c atom) + 2×6 (2 o atoms) = 16. We place three lone pairs of electrons around each f atom, accounting for 36 electrons. Since we have two oxygen atoms in o2, we multiply the number of valence electrons by 2, giving us a total of 12 valence electrons. 1st attempt part 1 (1 point) draw the lewis structure for o2.
Two Electrons Remain, And This Lone Pair Is Placed On The Xe Atom:
According to the octet rule, oxygen atoms need to bond twice. Web draw a skeleton structure of the molecule. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. O2 is also called oxygen gas (diatomic oxygen).
Web The Lewis Structure Of Xef 2 Shows Two Bonding Pairs And Three Lone Pairs Of Electrons Around The Xe Atom:
Web i quickly take you through how to draw the lewis structure of o2 (oxygen). The o 2 lewis structure has a double bond between two oxygen atoms. Drawing the lewis structure for o 2 ( dioxygen or oxygen gas) Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons.