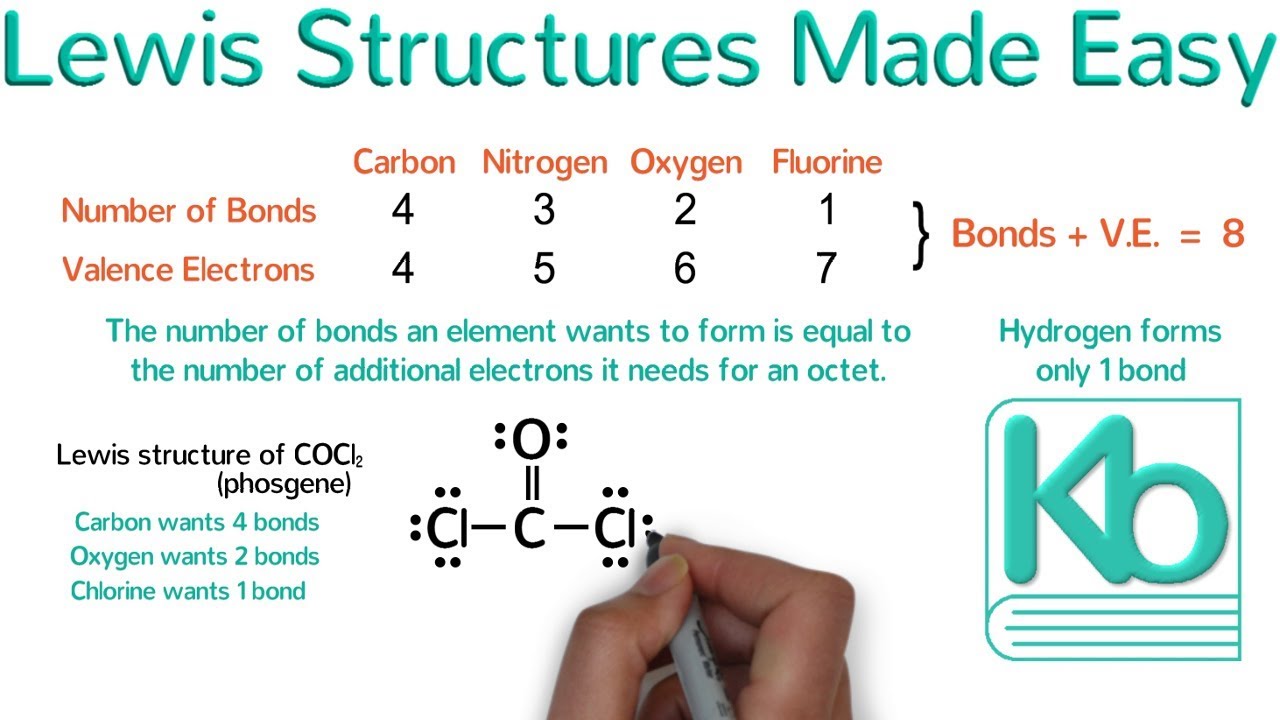
Draw The Lewis Dot Diagram For A H+ Cation
Draw The Lewis Dot Diagram For A H+ Cation - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Figure \(\pageindex{1}\) shows the lewis symbols for the elements of the third period of the periodic table. Draw the lewis dot diagram for a h+ cation make sure charges are in the diagram. Why would tuck first about a hydrogen atom?… A lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:
H+ valence electrons valence electrons are the electrons in the outermost shell of an atom. Hydrogen is in group 1 of the periodic table, so it has one valence electron. For hydrogen, it's just h. Having lost its two original valence electrons, the lewis electron dot diagram is just ca 2+. Draw the lewis dot diagram for a h+ cation make sure charges are in the diagram. Why would the hydrogen atom be the first to be touched? Web draw the lewis dot diagram for a h+ cation.
3.2 Lewis Dot Diagrams Chemistry LibreTexts
View more transcript video answer: Why would the hydrogen atom be the first to be touched? A lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. This problem has been solved! Having.
How to Draw a Lewis Structure
Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web we want to draw the lewis dot structure for the hydrogen ion h plus ions. Web we use lewis symbols to describe valence electron configurations of atoms and monatomic.
How to Draw Lewis Dot Structure
Web what is the lewis electron dot diagram for each ion? To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. The o 2− ion has gained two electrons in its valence shell, so its lewis electron dot diagram is as follows: Since h+ has.
Lewis Structures Made Easy Examples and Tricks for Drawing Lewis Dot
H+ valence electrons valence electrons are the electrons in the outermost shell of an atom. The o 2− ion has gained two electrons in its valence shell, so its lewis electron dot diagram is as follows: С с c 119 х $ ? Web draw the lewis dot diagram for a h+ cation make sure.
Lewis Structure Types
This problem has been solved! Why would the hydrogen atom be the first to be touched? Draw the lewis dot diagram for a h+ cation make sure charges are in the diagram. Ca 2+ the o 2− ion has gained two electrons in its valence shell,. Web draw a lewis electron dot diagram for an.
How to Draw a Lewis Structure
Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web draw the lewis dot diagram for a h+ cation. This problem has been solved! Web draw the lewis dot diagram for a h' cation. Since h+ has lost one electron, it has zero valence electrons. Web 01:50 draw the lewis.
Lewis Dot Structure Definition, Examples, and Drawing
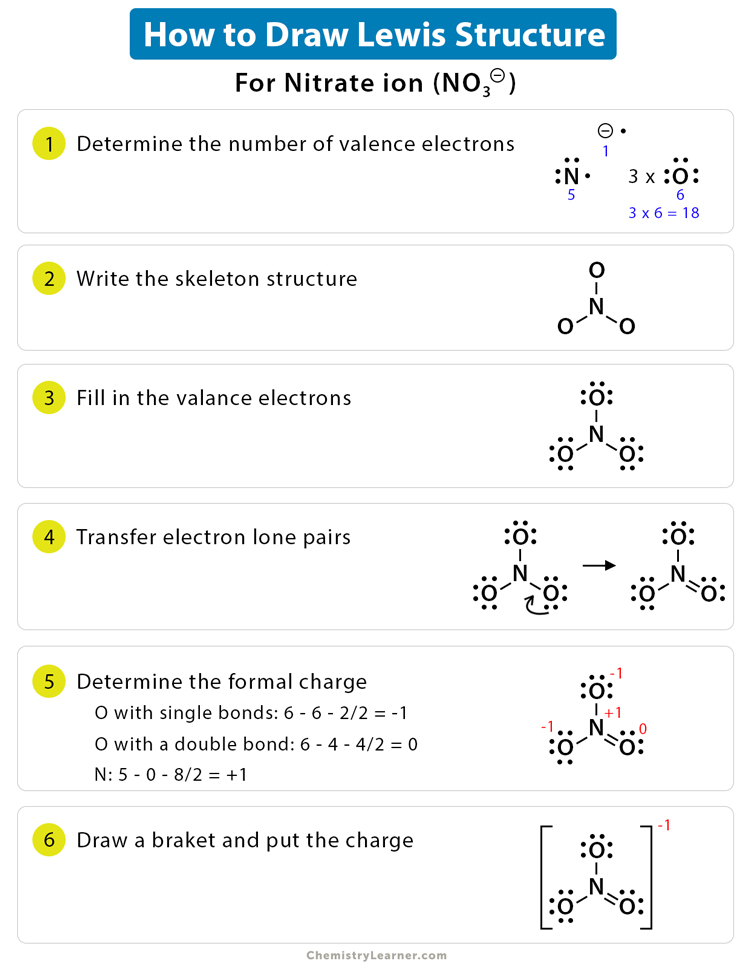
Web draw the lewis dot diagram for an as+ cation. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web so what would be the lewis dot structure for the compound of n2o2? Ca 2+ the o 2− ion has gained two electrons in its valence shell,. Draw.
Lewis Dot Structures of Atoms and Ions YouTube
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web 01:50 draw the lewis dot diagram for a c cation. Having lost its two original valence electrons, the lewis electron dot diagram is just ca 2+. Draw the lewis dot diagram for a h+ cation make sure charges.
How to Draw the Lewis Dot Structure for H+ (Hydrogen ion) YouTube
When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. In the periodic table, hydrogen is in group one of the periodic table. View more transcript video answer: I understand the valence electron part of it, but i don't understand the actual drawing of the lewis structure. Web to.
3 Ways to Draw Lewis Dot Structures wikiHow
I understand the valence electron part of it, but i don't understand the actual drawing of the lewis structure. For hydrogen, it's just h. Once we know how many valence electrons there are in h we can. H+ valence electrons valence electrons are the electrons in the outermost shell of an atom. Web to draw.
Draw The Lewis Dot Diagram For A H+ Cation For hydrogen, it's just h. The number of dots equals the number of valence electrons in the atom. Why would the hydrogen atom be the first to be touched? In this question, we want to draw the lewis dot structure for the hydrogen ion h plus ions. More related organic chemistry questions with video solutions
Having Lost Its Two Original Valence Electrons, The Lewis Electron Dot Diagram Is Just Ca 2+.
The number of dots equals the number of valence electrons in the atom. Having lost its two original valence electrons, the lewis electron dot diagram is just ca 2+. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. To do that, we take the chemical symbol.
This Problem Has Been Solved!
Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Ca 2+ the o 2− ion has gained two electrons in its valence shell,. The number of dots equals.
More Related Organic Chemistry Questions With Video Solutions
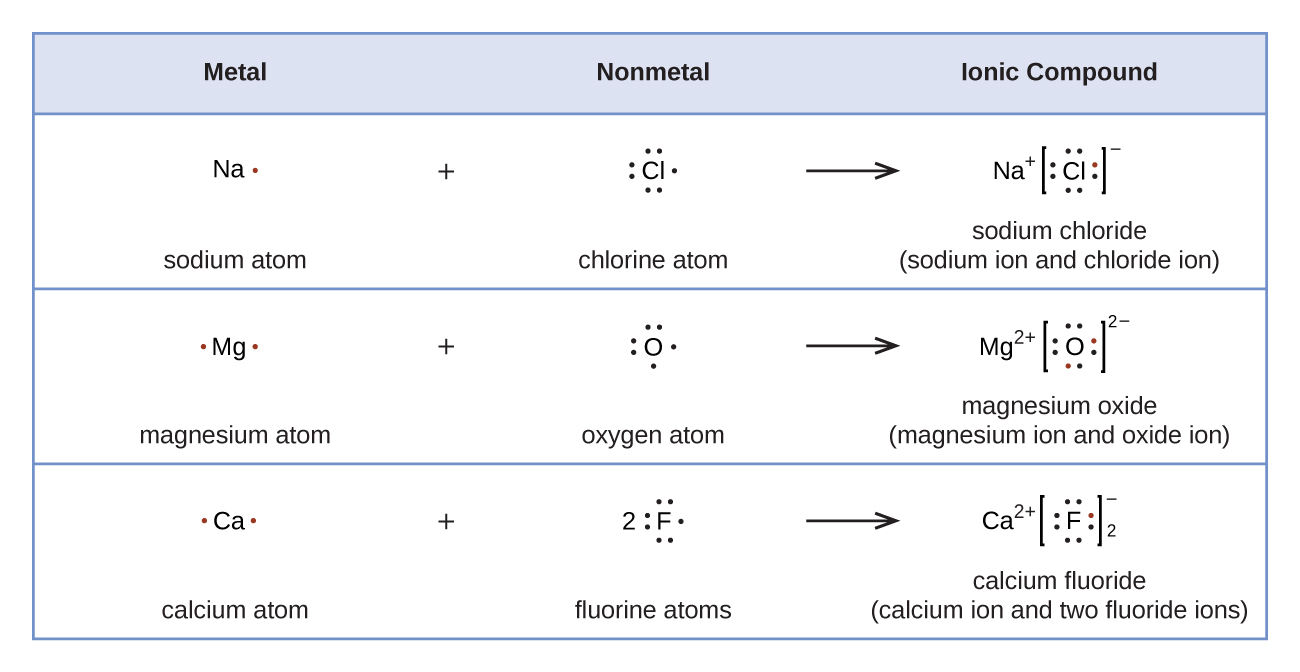
Web draw the lewis dot diagram for a h+ cation make sure charges are in the diagram this problem has been solved! We now see that each o atom needs 2 electrons to make up an octet, while each al atom has 3 electrons to donate. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. This problem has been solved!
In The Periodic Table, Hydrogen Is In Group One Of The Periodic Table.
Web draw a lewis electron dot diagram for an atom or a monatomic ion. I show you where hydrogen is on the periodic table. Web to draw the lewis structure of h+, we need to consider the valence electrons of hydrogen. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.

/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)