Draw The Electron Configuration For A Neutral Atom Of Silicon
Draw The Electron Configuration For A Neutral Atom Of Silicon - Web what is the electron configuration of a neutral chlorine atom? Remove electrons from the highest shell, starting with the highest energy subshell. The p, d, and f orbitals have different sublevels, thus can hold more electrons. Therefore, it would have enough electrons to fill up till its 3p shell. This problem has been solved!
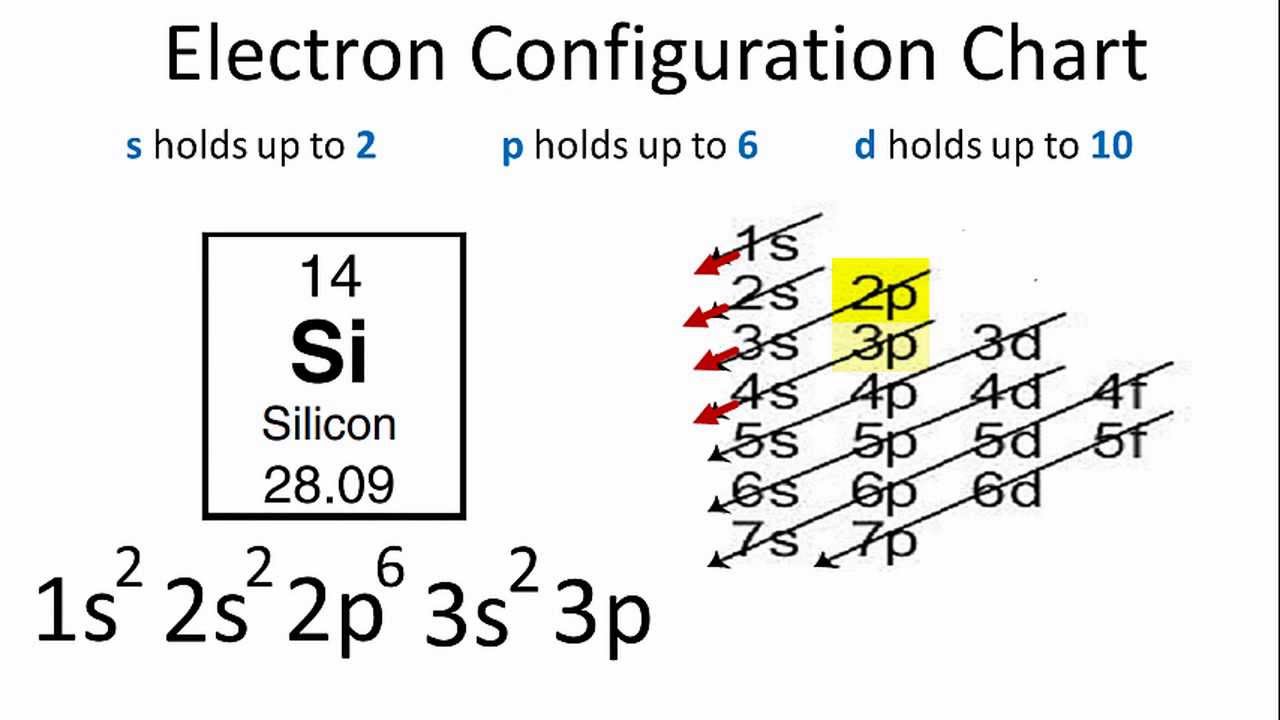
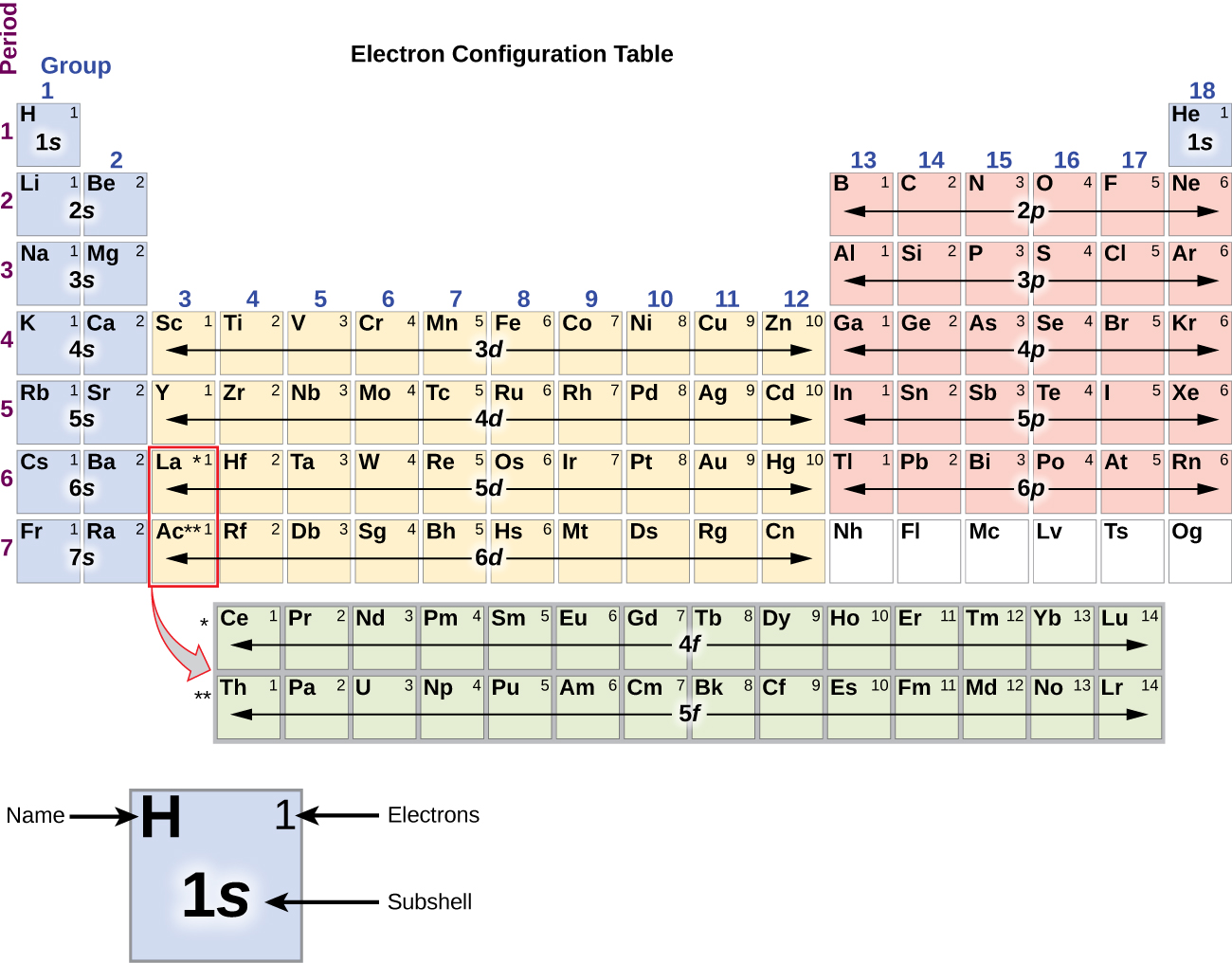
Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. The electron configuration of silicon is [ ne] 3s 2 3p 2 , if the electron arrangement is through orbitals. Web the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal. Determine how many electrons were lost. An atom has a valence shell electron configuration of #ns^1#. Remember, a neutral atom contains the same number of protons and electrons. When we write the configuration we'll put all 14 electrons in orbitals around the nucleus of the silicon atom.
Electron Configuration Chart With Orbitals
Therefore, it would have enough electrons to fill up till its 3p shell. The outer valance electron configuration of # s^2 p^2 allows silicon to have common charges of +2 and +4. Web the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous.
Silicon Atom Science Notes and Projects
The atomic number of cl is 17. 1s^2 2s^2 2p^6 3s^2 3p^2 silicon has a similar electron configuration to carbon. The outer valance electron configuration of # s^2 p^2 allows silicon to have common charges of +2 and +4. I show you where silicon is on the periodic table and how to determine how many.
Silicon Electron Configuration (Si) with Orbital Diagram
Draw the electron configuration for a neutral atom of silicon. Web typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. 1s 2 2s 2 2p 6 3s 2 3p 0. Web what is the electron.
Electron arrangements
1s 2 2s 2 2p 6 3s 2 3p 0. Web march 23, 2023 by jay electron configuration chart of all elements is mentioned in the table below. There are three rules followed for drawing the orbital diagram for an atom. The electron configuration of silicon is [ ne] 3s 2 3p 2 , if.
Silicon Electron Configuration Orbital Diagram For Silicon (Si)
This problem has been solved! Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. 5 10 11 12 13 14 draw the electron configuration for a neutral atom of silicon energy x subu alignment continue giv #1s^2, 2 s^2, 2p^6, 3s^2,.
Silicon Electron Configuration (Si) with Orbital Diagram
Remember, a neutral atom contains the same number of protons and electrons. Web what is the electron configuration of a neutral chlorine atom? An atom has a valence shell electron configuration of #ns^1#. What is the name of this atom? Let's draw the electron configuration for a neon neon, has a neutral atom has 10.
Silicon Si (Element 14) of Periodic Table Elements FlashCards
But wait — you can avoid all this if you use our tool! Determine how many electrons were lost. Web an electrically neutral atom has the following electron configuration: The electron configuration of silicon is [ ne] 3s 2 3p 2 , if the electron arrangement is through orbitals. This problem has been solved! Web.
Symbol and electron diagram for silicon Royalty Free Vector
1s^2 2s^2 2p^6 3s^2 3p^2 silicon has a similar electron configuration to carbon. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the electron configuration for a neutral atom of silicon. Remember, a neutral atom contains the same number of protons and electrons. Energy 1 1 х.
Electronic Configurations Intro Chemistry LibreTexts
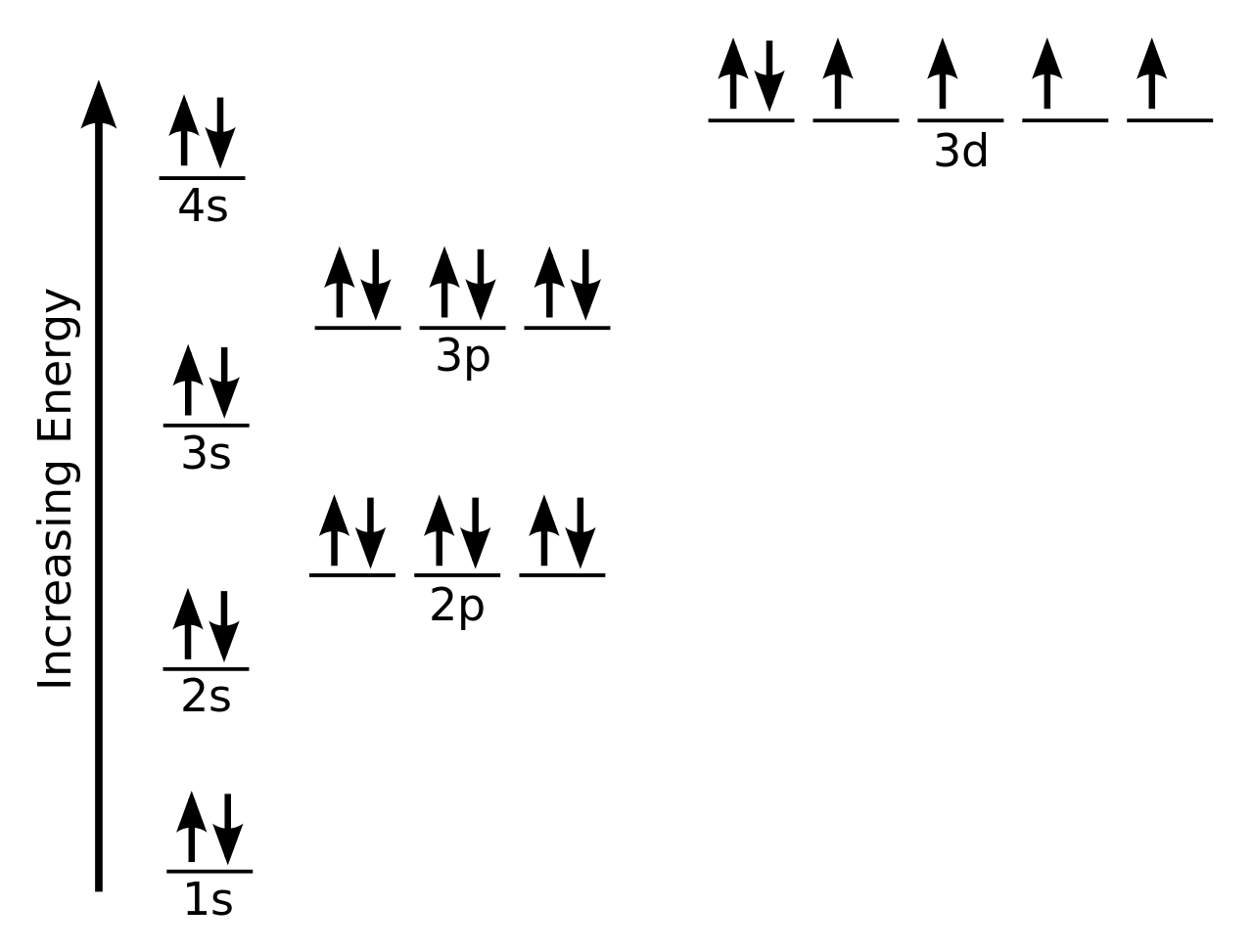
This problem has been solved! But wait — you can avoid all this if you use our tool! Let's draw the electron configuration for a neon neon, has a neutral atom has 10 electrons, so we are going to do this in terms of energy, increasing energy on the y axis and 1 s, 2.
Silicon Electron Configuration How Many Unpaired Electrons Ellis
#1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. The atomic number of cl is 17. But wait — you can avoid all this if you use our tool! 1s 2 2s 2 2p 6 3s 2 3p 0. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web in order.
Draw The Electron Configuration For A Neutral Atom Of Silicon Remove electrons from the highest shell, starting with the highest energy subshell. Web the four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Write the configuration of the neutral atom. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell.
1S 2 2S 2 2P 6 3S 2 3P 1.
As stated, the electron configuration of each element is unique to its position on the periodic table. An atom has a valence shell electron configuration of #ns^1#. Web the arrangement of electrons in silicon in specific rules in different orbits and orbitals is called the electron configuration of silicon. Write the configuration of the neutral atom.
Web The Four Different Types Of Orbitals (S,P,D, And F) Have Different Shapes, And One Orbital Can Hold A Maximum Of Two Electrons.
Web what is the electron configuration of a neutral chlorine atom? Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Web a silicon atom is a neutral atom that has an atomic number of 14 which implies it has a total of 14 electrons. 5 10 11 12 13 14 draw the electron configuration for a neutral atom of silicon energy x subu alignment continue giv
Therefore, Its Ground State Electronic Configuration Can Be Written As 1S 2 2S 2 2P 6 3S 2 3P 5.
Remember, a neutral atom contains the same number of protons and electrons. But wait — you can avoid all this if you use our tool! Energy 1 1 х ? Si 4 + was formed by the loss of four electrons.
1S^2 2S^2 2P^6 3S^2 3P^2 Silicon Has A Similar Electron Configuration To Carbon.
Draw the electron configuration for a neutral atom of silicon. Web the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.