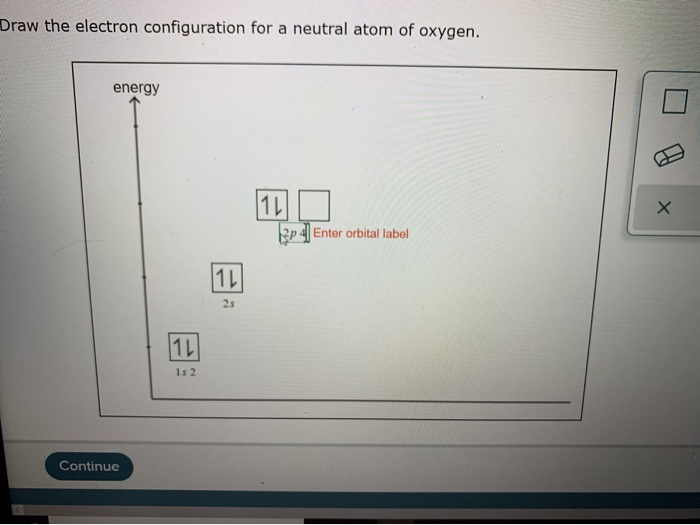
Draw The Electron Configuration For A Neutral Atom Of Oxygen
Draw The Electron Configuration For A Neutral Atom Of Oxygen - Valence electrons are the electrons in the outermost shell, or energy level, of an atom. 100% (2 ratings) step 1. Web electron configuration of boron (b) [he] 2s 2 2p 1: Web elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). The given atom is neutral.
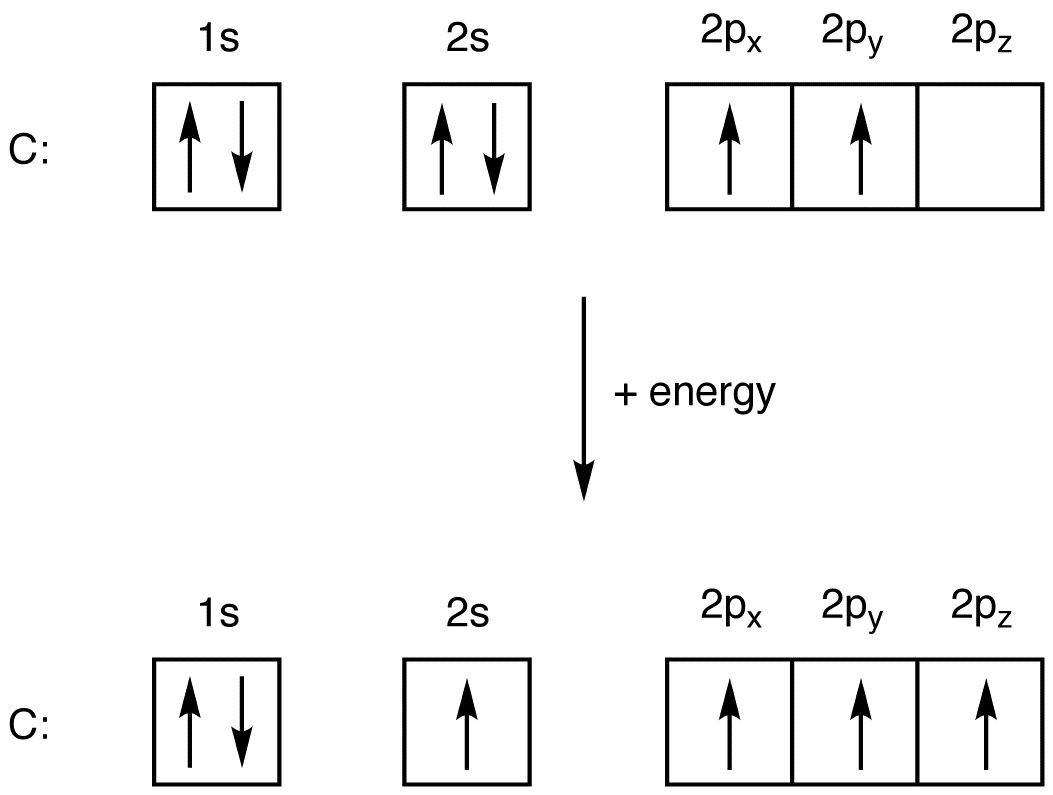
1s 2 2s 2 2p 6 3s 2 3p 1. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. Web an electrically neutral atom has the following electron configuration: 1s 2 2s 2 2p 6 3s 1. This electron configuration of oxygen shows that the outer. The remaining four electrons will go in the 2p orbital. View the full answer step 2.
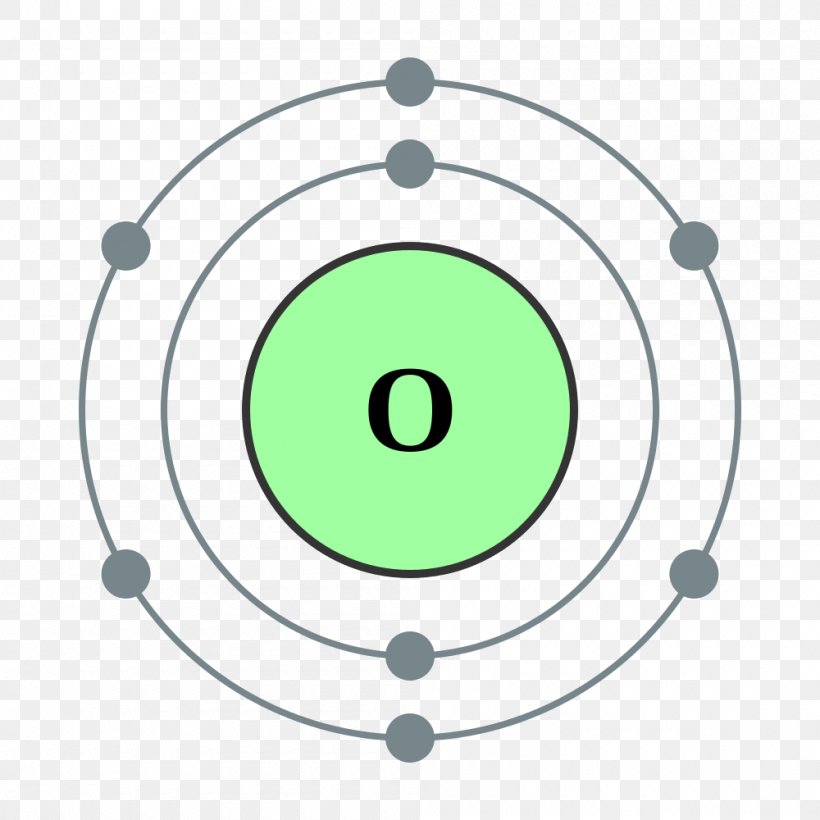
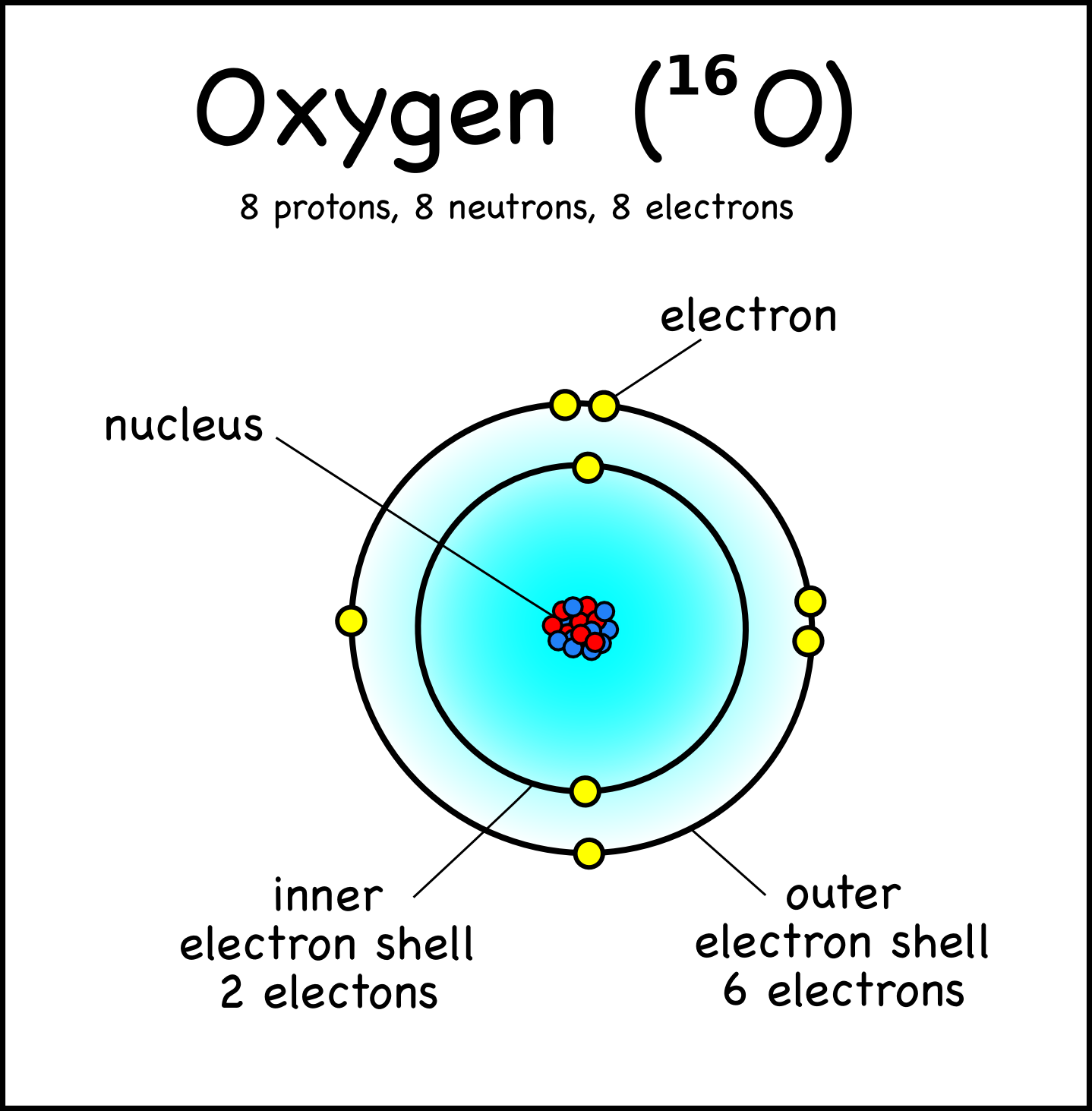
Bohr Model Oxygen Chemical Element Atomic Number, PNG, 1000x1000px
Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. View the full answer step 2. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. 1s 2 2s 2 2p 2: For example, the electron configuration.
Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
This electron configuration of oxygen shows that the outer. Web if we look at the element after nitrogen in the same period, oxygen (z = 8) its electron configuration is: The given element is oxygen ( o ). Electron configuration of oxygen (o) [he] 2s 2 2p 4: Electron configuration of nitrogen (n) [he] 2s.
Solved Draw the electron configuration for a neutral atom of
We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon atom lacks an octet: 1s 2 2s 2 2p 6 3s 1 However, it's easy to determine the configuration of electrons for heavier elements by making a chart. The colors of an aurora are attributed to electron transitions in.
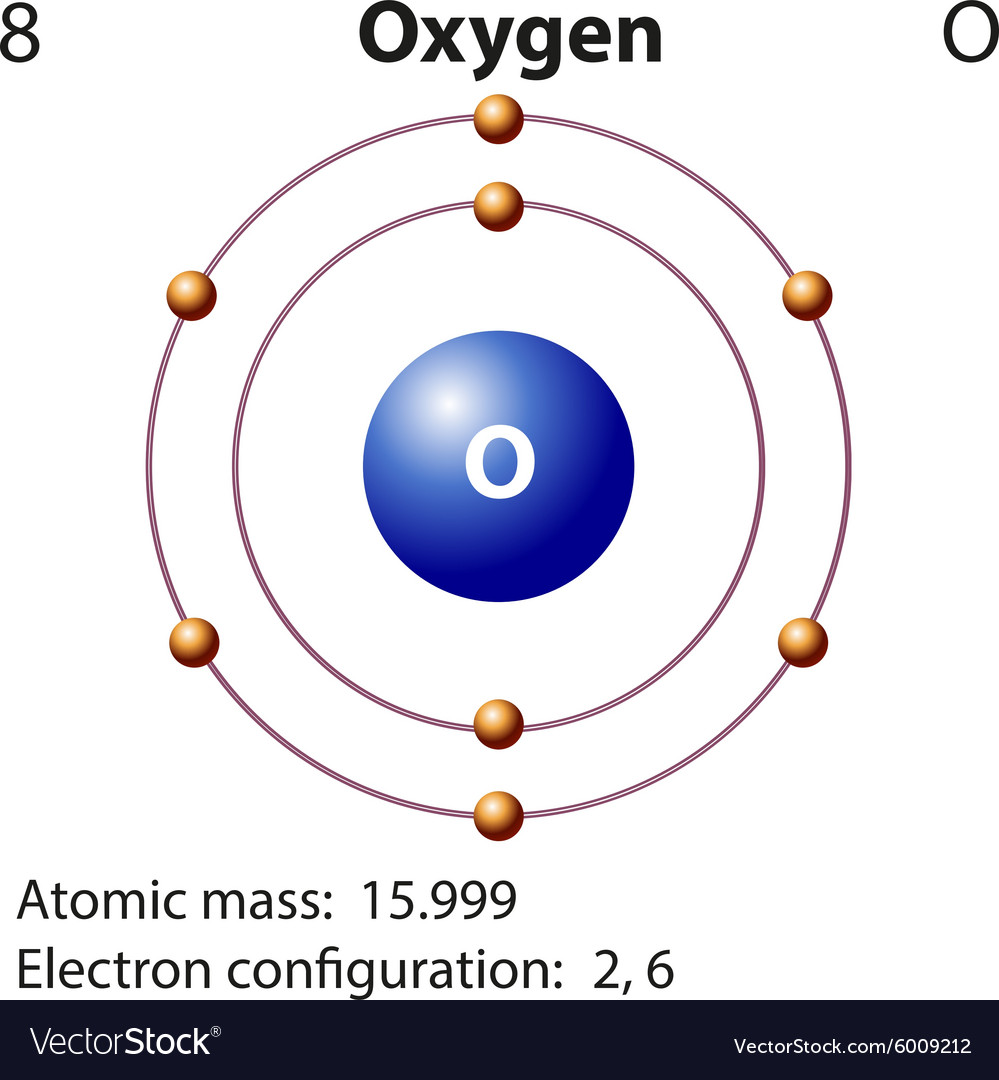
How to Write Ground State Electron Configuration in Chemistry
This allows each halogen atom to have a noble gas electron configuration. Web the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively,.
Diagram representation element oxygen Royalty Free Vector
1s 2 2s 2 2p 6 3s 2 3p 1. The atomic number of oxygen ( o) is 8. For example, the electron configuration of oxygen looks like: 1s 2 2s 2 2p 6 3s 2 3p 2. The electron configuration of oxygen is [ he] 2s 2 2p 4, if the electron arrangement is.
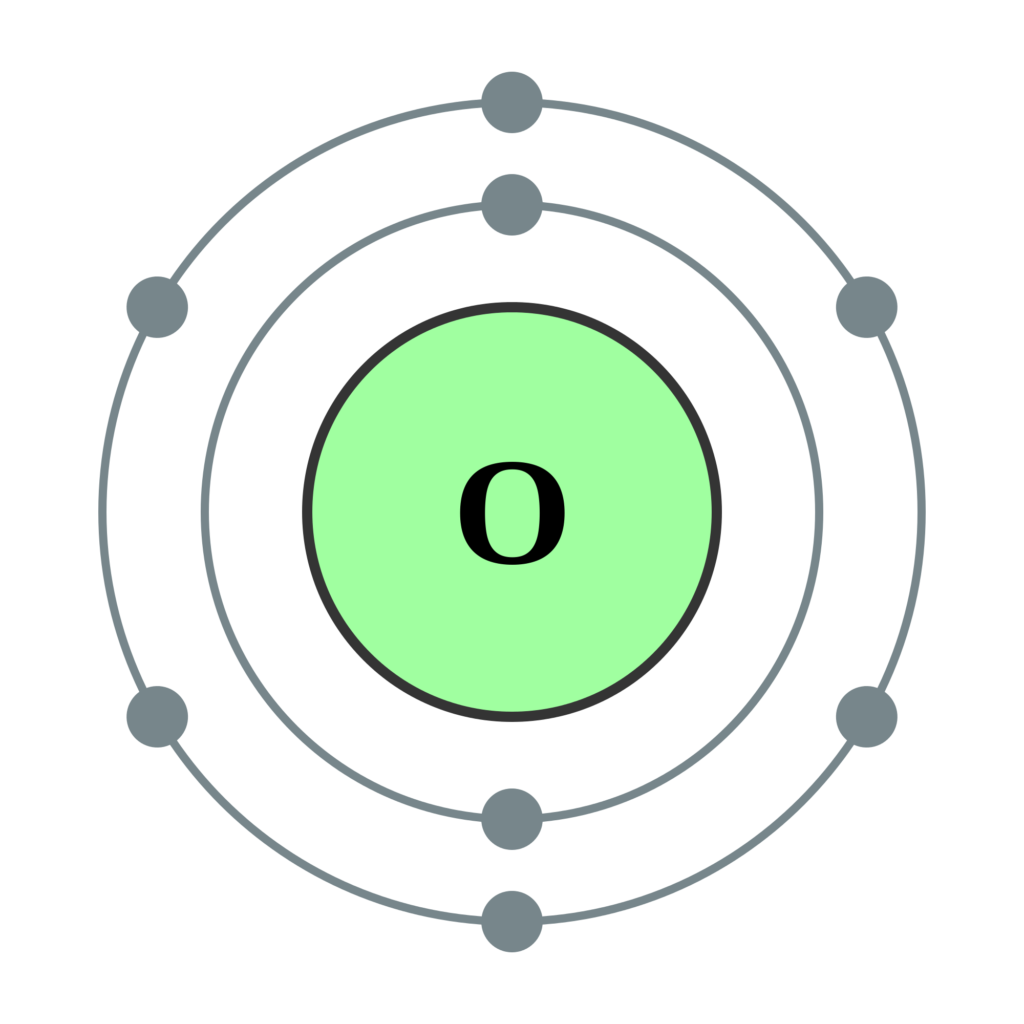
Oxygen Electron Configuration (O) with Orbital Diagram
View the full answer step 2. The given element is oxygen ( o ). 1s 2 2s 2 2p 4: Web oxygen, for example, has the electron configuration 1s 2 2s 2 2p 4, whereas the oxygen anion has the electron configuration of the noble gas neon (ne), 1s 2 2s 2 2p 6. An.
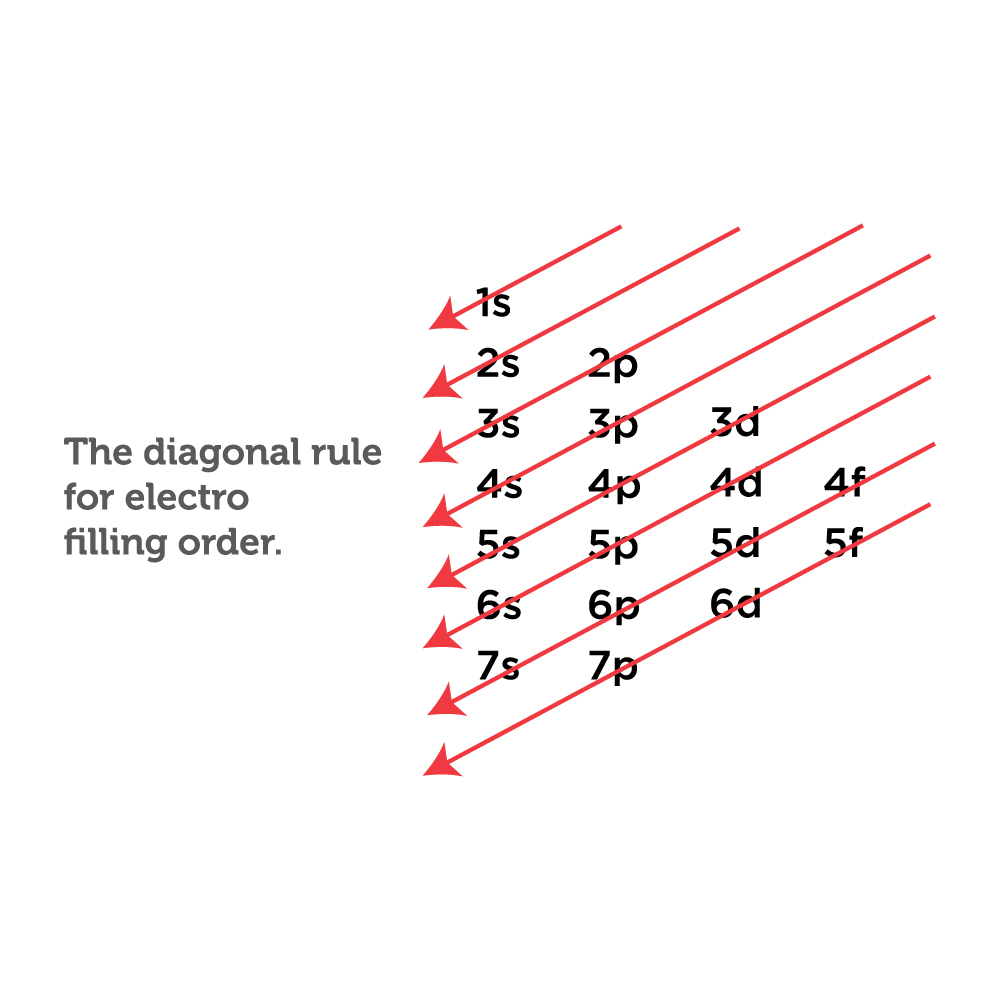
Electron Configuration Chart With Orbitals
Draw lewis structures depicting the bonding in simple molecules; Remove electrons from the highest shell, starting with the highest energy subshell. An atom has a valence shell electron configuration of #ns^1#. The given atom is neutral. Determine how many electrons were lost. Electron configuration can be done in two ways. The electron configuration of oxygen.
Drawing Atoms Montessori Muddle
Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. What is the name of this atom? Electron configuration of carbon (c) [he] 2s 2 2p 2: Remove electrons from the highest shell, starting with the highest energy subshell. An atom has a.
Forms of Energy ND Studies Energy Curriculum
For example, the electron configuration of oxygen looks like: Web with three unpaired electrons. How many core electrons are there? 1s 2 2s 2 2p 1: This means the first shell (1s) has 2. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. Web the electronic configuration of anions.
What is the Electron Configuration of Oxygen Archives Dynamic
However, it's easy to determine the configuration of electrons for heavier elements by making a chart. And valence electrons are those electrons found in the outer shell of an atom. This electron configuration of oxygen shows that the outer. In order to write the o electron configuration we first need to know t. Web oxygen.
Draw The Electron Configuration For A Neutral Atom Of Oxygen Web write lewis symbols for neutral atoms and ions; The atomic number of oxygen ( o) is 8. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. Since 1s can only hold two electrons the next 2 electrons for o go in the 2s orbital. The electron configuration of nitrogen is thus 1 s2 2 s2 2 p3.
Web Write Lewis Symbols For Neutral Atoms And Ions;
Electron configuration of oxygen (o) [he] 2s 2 2p 4: Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. 100% (2 ratings) step 1. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle.
This Means The First Shell (1S) Has 2.
The idealized pes spectrum of oxygen. From the pauli exclusion principle, we know that an orbital can contain two electrons with. The electron configuration of oxygen is [ he] 2s 2 2p 4, if the electron arrangement is through orbitals. The electron configuration of nitrogen is thus 1 s2 2 s2 2 p3.
Web Because Chemists Are Really Interested In Keeping Track Of Where All The Electrons In A Given Atom Live, They Write Down A Series Of Symbols Called An Electron Configuration That Keeps Track Of All Of This Information For A Given Atom.
However, it's easy to determine the configuration of electrons for heavier elements by making a chart. Electron configuration of carbon (c) [he] 2s 2 2p 2: Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. What is the name of this atom?
1S 2 2S 2 2P 4:
#1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. Since 1s can only hold two electrons the next 2 electrons for o go in the 2s orbital. Web if we look at the element after nitrogen in the same period, oxygen (z = 8) its electron configuration is: 1s 2 2s 2 2p 1: