Draw The Electron Configuration For A Neutral Atom Of Fluorine
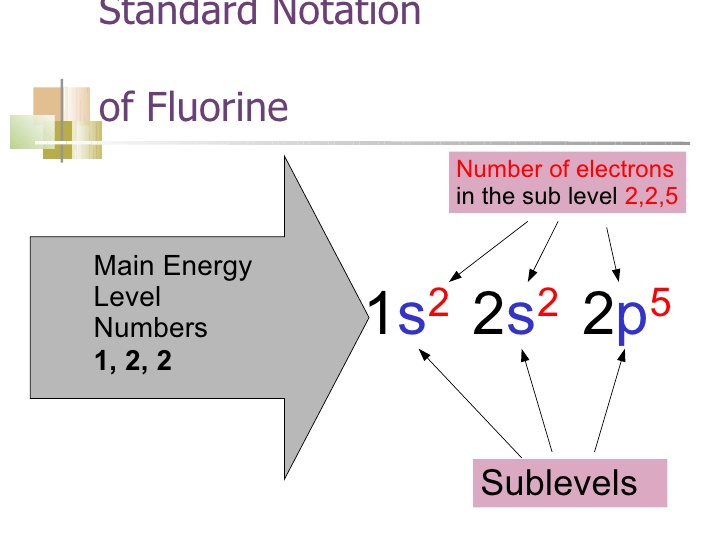
Draw The Electron Configuration For A Neutral Atom Of Fluorine - Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Electron configuration of fluorine (f) [he] 2s 2 2p 5: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3. 1s 2 2s 2 2p 3:
Web you can check it immediately in our valence electron calculator on the left or look at the position of a particular atom on the periodic table, which will help you to determine how many valence electrons a neutral atom of that element has. Electron configuration of oxygen (o) [he] 2s 2 2p 4: Energy х this problem has been solved! Electron configuration of fluorine (f) [he] 2s 2 2p 5: Draw an orbital diagram and use it to derive the electron configuration of phosphorus, z = 15. Draw the electron configuration for a neutral atom of fluorine. Web its electron configuration will be f:
How many valence electrons does fluorine(F) have?
Web write the full electron configuration for a neutral fluorine atom. Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of fluorine. Because all the 2p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. Web you can check it immediately in our valence electron.
Periodic Table Fluorine Valence Electrons Periodic Table Timeline
Question what is the electron configuration of fluorine? Fluorine belongs to group 17 which is known as halogens. Web electron configuration for fluorine electron configuration notation: Therefore, the number of electrons in neutral atom of fluorine is 9. 1s 2 2s 2 2p 3: Web science chemistry chemistry questions and answers draw the electron configuration.
fluorine orbital diagram Herbalned
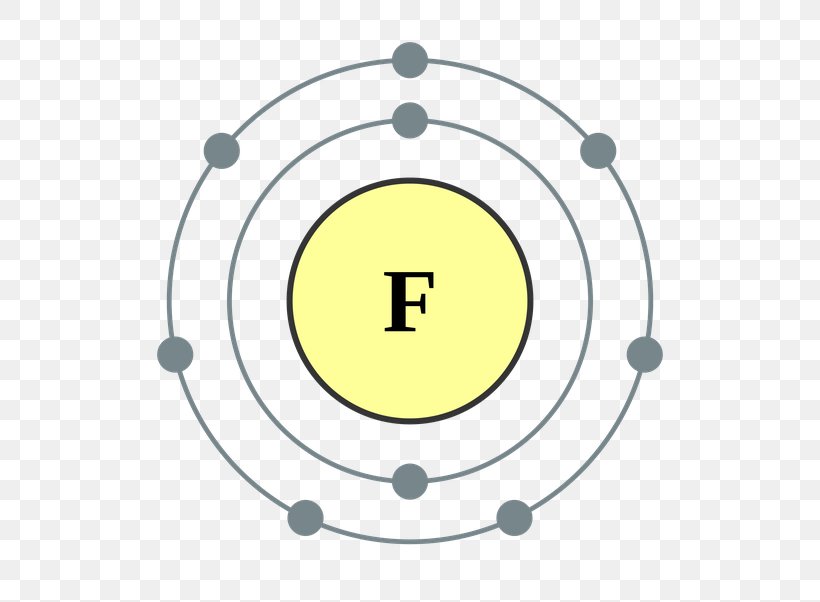
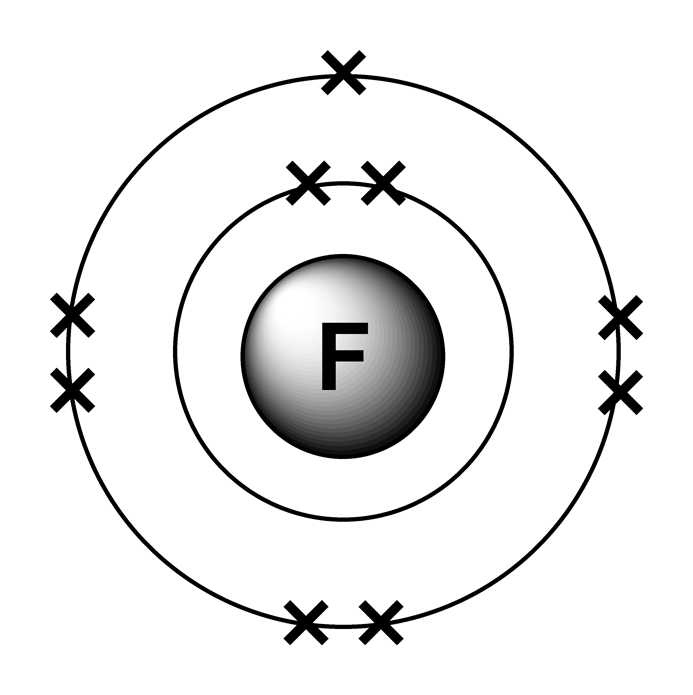
The first 2 electrons are found in the first energy level, and the other 7 are found in the second energy level. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Write the full electron configuration for a neutral fluorine atom. Web the arrangement of electrons in fluorine in specific rules in different orbits.
Diagram representation element fluorine Royalty Free Vector
Which means that the electron configuration given to you corresponds to a fluorine atom in an excited state. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. First ionisation energy the minimum.
How To Find Electron Configuration For Fluorine Dynamic Periodic
A slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). 1s 2 2s 2 2p 4: Web fluorine has one electron pair in the 1 s orbital, one electron pair in the 2 s orbital, and 2 electrons pairs with one unpaired electron in the 2 p orbital..
Fluorine F (Element 9) of Periodic Table Elements Flash Cards
All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n= 1 and the n= 2 shells are filled. The neutral atom chlorine (z=17), for instance has 17 electrons. All of the electrons in the noble gas neon (atomic number 10) are paired, and.
Fluorine Electron Configuration YouTube
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question what is the electron configuration of fluorine? The electron configurations and orbital diagrams of these four elements are: First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. Web write the.
Electron configurations
Question what is the electron configuration of fluorine? Draw the electron configuration for a neutral atom of fluorine. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5 and the orbital diagram is: When we reach.
The Electron Configuration of Fluorine YouTube
Web electron configuration of nitrogen (n) [he] 2s 2 2p 3: Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. The first 2 electrons are found in the first energy level, and.
Fluorine atom hires stock photography and images Alamy
1s 2 2s 2 2p 3: We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. When we reach neon, with z = 10, we have filled the 2 p subshell, giving a 1 s 2 2 s 2 2 p 6 electron configuration:.
Draw The Electron Configuration For A Neutral Atom Of Fluorine A slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. Web fluorine has one electron pair in the 1 s orbital, one electron pair in the 2 s orbital, and 2 electrons pairs with one unpaired electron in the 2 p orbital. Web fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Draw the electron configuration for a neutral atom of fluorine.
Therefore, The Number Of Electrons In Neutral Atom Of Fluorine Is 9.
Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. Web electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. Draw an orbital diagram and use it to derive the electron configuration of phosphorus, z = 15.
The Elements Of Group 17 Have Seven Electrons In Their Outermost Shell.
How to write the electron configuration for fluorine Web write the full electron configuration for a neutral fluorine atom. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3. Energy х this problem has been solved!
The Periodic Table Gives The Following Electron Configuration:
When we reach neon, with z = 10, we have filled the 2 p subshell, giving a 1 s 2 2 s 2 2 p 6 electron configuration: 1s 2s22p draw the lewis dot symbol for a neutral fluroine atom identify the subshells in the full electron configuration whose electrons are included in the lewis dot symbol for the neutral fluorine atom सी 3p 1x 1 fif: Web this electron configuration corresponds to a neutral atom of fluorine, f. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals.
1S22S22P5 Draw The Lewis Dot Symbol For A Neutral Fluroine Atom.
Which means that the electron configuration given to you corresponds to a fluorine atom in an excited state. Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5 and the orbital diagram is: Web in this case, 2+2+6+2+6+2+10+6+2+1= 39 and z=39, so the answer is correct. Draw the electron configuration for a neutral atom of fluorine.