Draw The Electron Configuration For A Neutral Atom Of Cobalt
Draw The Electron Configuration For A Neutral Atom Of Cobalt - Web the electron configuration of cobalt is [ ar] 3d 7 4s 2 , if the electron arrangement is through orbitals. An ion of an atom is one in which the number of protons and. The cobalt (iii) ion, however, has lost 3 electrons, leaving it with 24 electrons. Electrons always try to fill the lowest energy levels available. 1s 2 2s 2 2p 3:
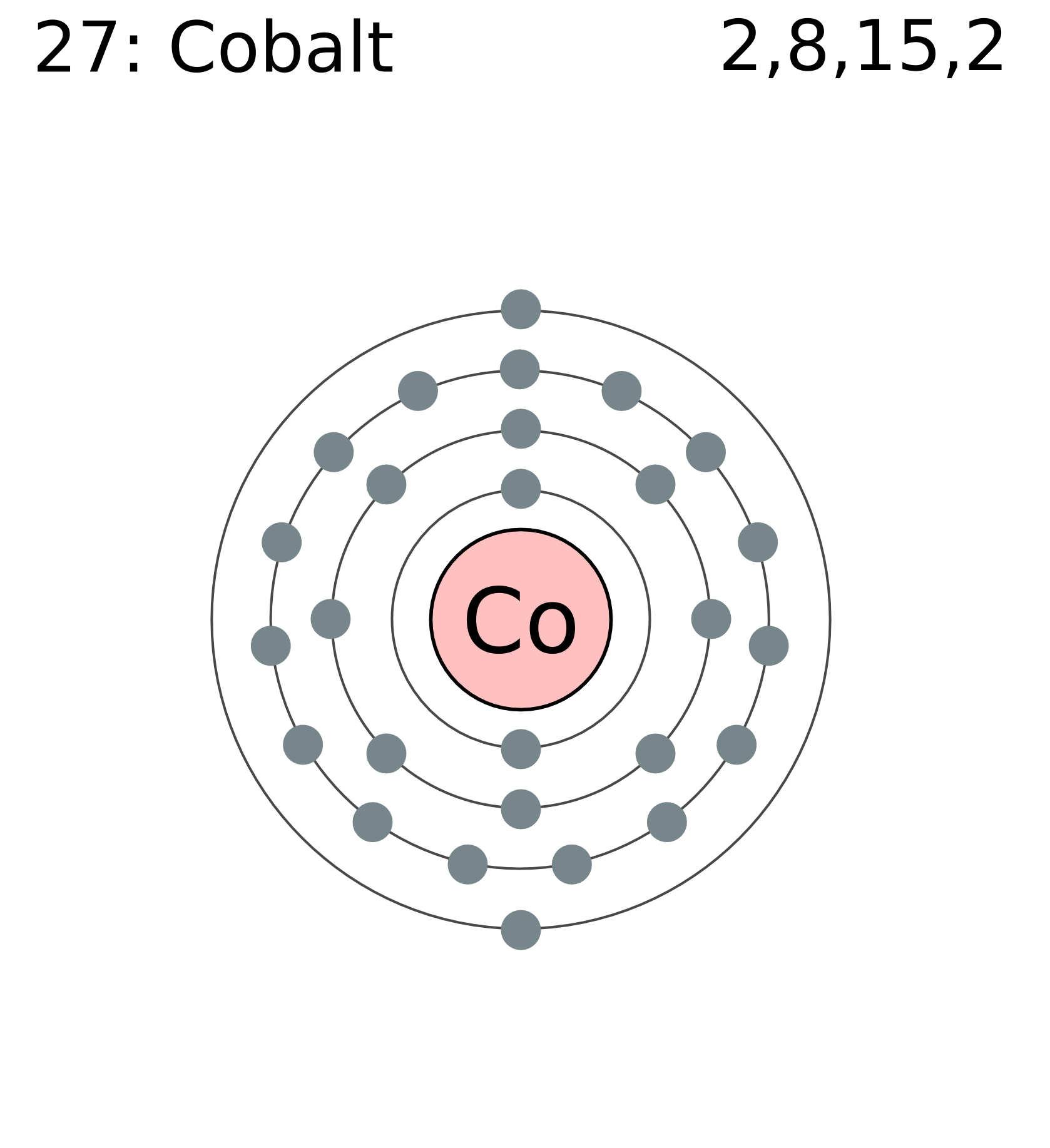
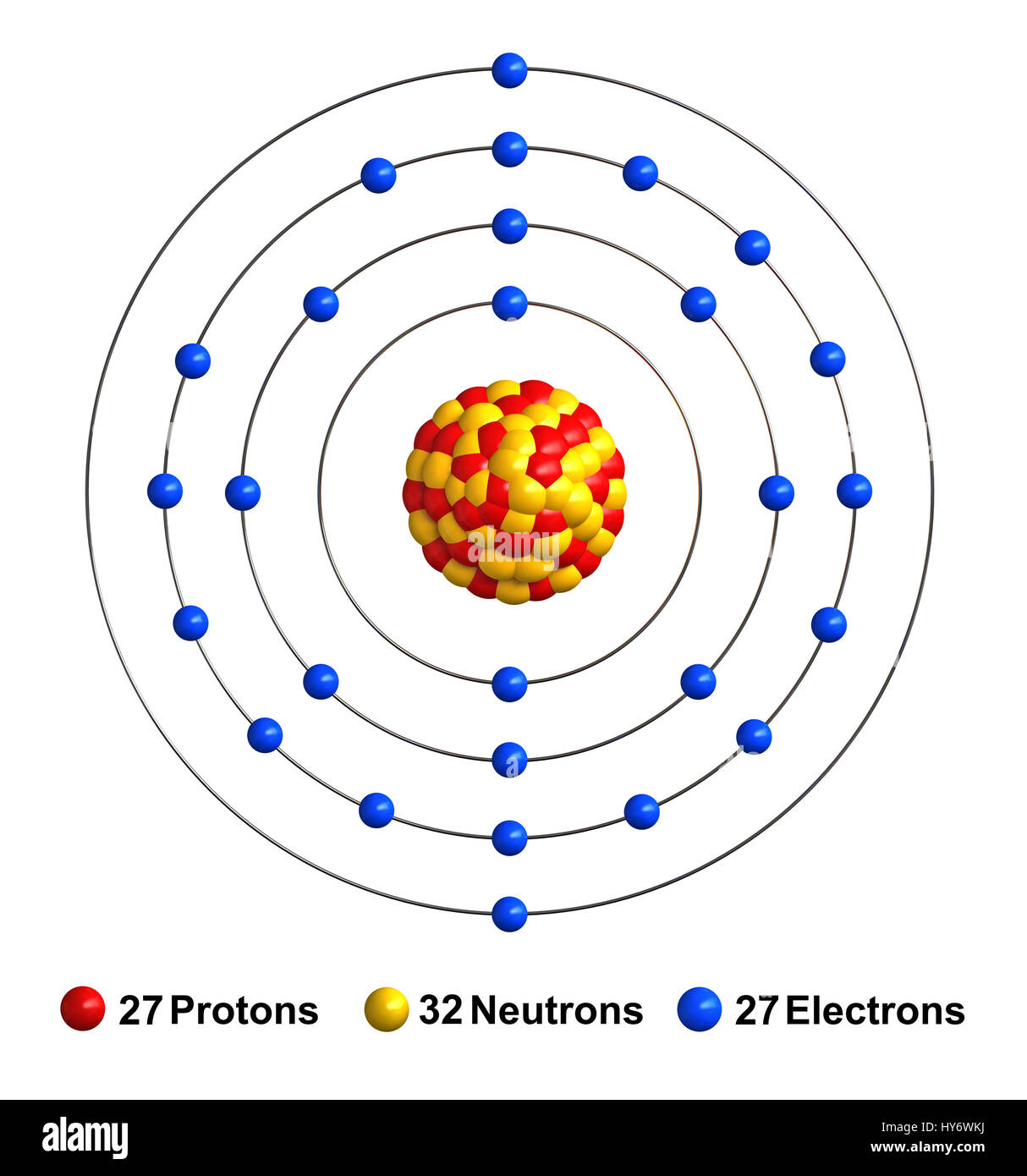
Since the atomic number is 27, there are 27 electrons in a neutral cobalt atom. Determine how many electrons were lost. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. When we write the configuration, we'll put all 27 electrons in orbitals around the nucleus of the cobalt atom. Web this electron configuration is written as 1 s2 2 s1. Chemistry electron configuration electron configuration 1 answer david drayer aug 22, 2016 cobalt atomic number 27 has 1s2 2s2 2p6 3s2 3p^6 4s^2 3d^7# explanation:
Cobalt Electron Configuration (Co) with Orbital Diagram
Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) cobalt (co) atom electron configuration (bohr model) Chemistry electron configuration electron configuration 1 answer david drayer aug 22, 2016 cobalt atomic number 27 has 1s2 2s2 2p6 3s2 3p^6 4s^2 3d^7# explanation: The next element is beryllium, with z = 4 and four.
Symbol and electron diagram for Cobalt illustration Stock Vector Image
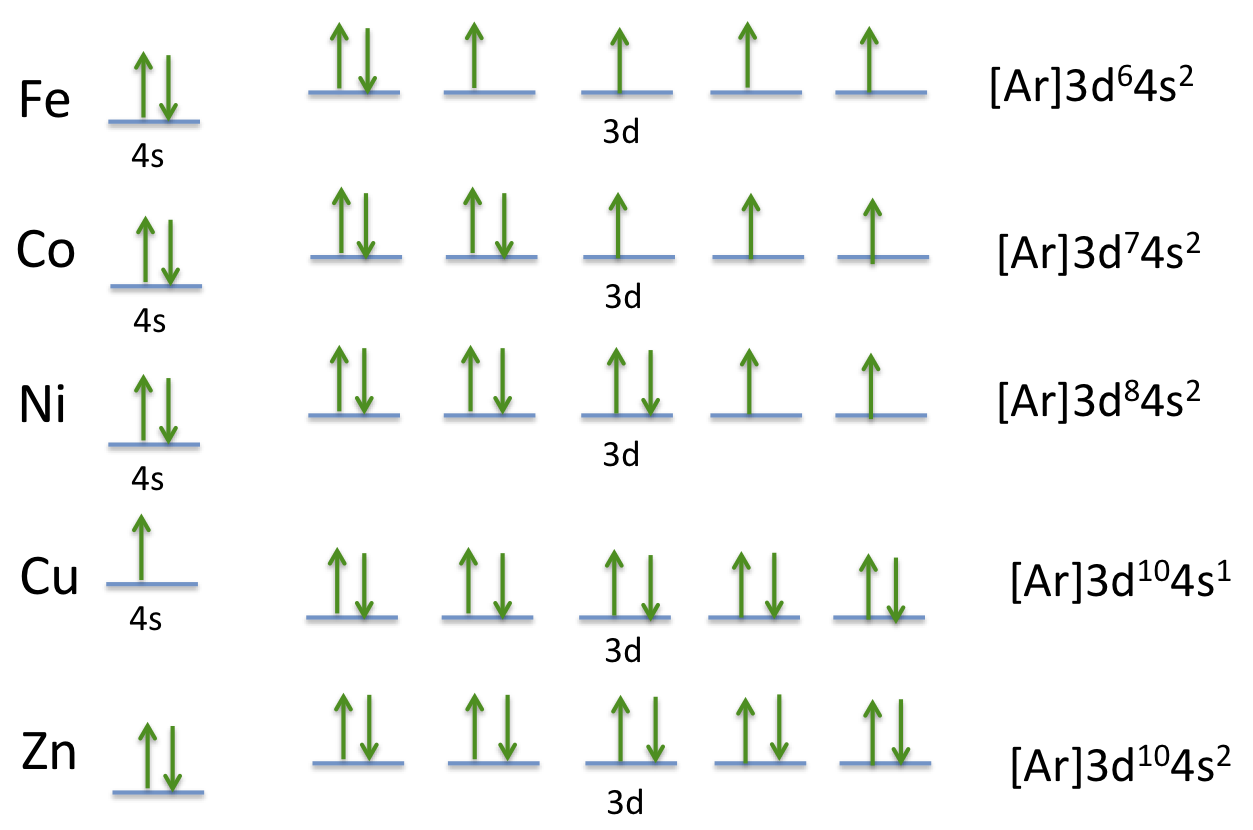
So, the electron configuration for a neutral atom of cobalt is: The electron configurations and orbital diagrams of these four elements are: When we reach boron, with z = 5 and five electrons, we must place the fifth electron in one of the 2 p orbitals. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁷ 4p¹. When.
Cobalt Definition, Facts, Symbol, Discovery, Property, Uses
1s 2 2s 2 2p 6 3s 1 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁷. When we write the configuration, we'll put all 27 electrons in orbitals around the nucleus of the cobalt atom. The neutral atom chlorine (z=17), for instance has 17 electrons. When we reach boron, with z = 5 and five electrons,.
A stepbystep description of how to write the electron configuration
Web this electron configuration is written as 1 s2 2 s1. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating. 1s 2 2s 2 2p 6 3s.
Chemist atom of cobalt diagram 366912 Vector Art at Vecteezy
Si 4 + was formed by the loss of four electrons. Write the configuration of the neutral atom. 1s 2 2s 2 2p 6 3s 1 Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n =.
draw the electron configuration for a neutral atom of cobalt
Therefore, the electron configuration of the cobalt (iii) ion is [ar] 4s1 3d5. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher.
Cobalt Electron Configuration (Co) with Orbital Diagram
Web science chemistry chemistry questions and answers give the electron configuration for a neutral atom of cobalt (co) using an orbital diagram (the kind with lines or boxes for orbitals and arrows to show the electrons). Web there are 27 electrons for the cobalt atom. Web a) draw and write the electron configuration for a.
3d render of atom structure of cobalt isolated over white background
Web there are 27 electrons for the cobalt atom. The next element is beryllium, with z = 4 and four electrons. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web a) draw and write the electron configuration for a neutral atom of cobalt: Therefore, the electron configuration of.
Solved Draw the electron configuration for a neutral atom of
Web the electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁷. Determine how many electrons were lost. An ion of an atom is one in which the number of protons and. Web the upper right side shows.
Cobalt Protons Neutrons Electrons Electron Configuration
Web this electron configuration is written as 1 s2 2 s1. The cobalt (iii) ion, however, has lost 3 electrons, leaving it with 24 electrons. When we write the configuration, we'll put all 27 electrons in orbitals around the nucleus of the cobalt atom. Web the electron configuration of a neutral cobalt atom is [ar].
Draw The Electron Configuration For A Neutral Atom Of Cobalt Web electron configuration of nitrogen (n) [he] 2s 2 2p 3: Remember, a neutral atom contains the same number of protons and electrons. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. When we reach boron, with z = 5 and five electrons, we must place the fifth electron in one of the 2 p orbitals. 1s 2 2s 2 2p 6 3s 2 3p 1.
Web Electron Configuration Of Nitrogen (N) [He] 2S 2 2P 3:
Write the configuration of the neutral atom. Web the electron configuration of a neutral cobalt atom is: So, the electron configuration for a neutral atom of cobalt is: When we reach boron, with z = 5 and five electrons, we must place the fifth electron in one of the 2 p orbitals.
The Neutral Atom Chlorine (Z=17), For Instance Has 17 Electrons.
Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating. However, to achieve a more stable configuration, one of the 4s electrons can be excited to the 3d level. Find the atomic number of cobalt from the periodic table. Therefore, the electron configuration of the cobalt (iii) ion is [ar] 4s1 3d5.
Since The Atomic Number Is 27, There Are 27 Electrons In A Neutral Cobalt Atom.
We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. A) draw and write the electron configuration for a neutral atom of cobalt. Check the atomic number (29) and atomic mass (63.546) of copper.
We Fill Both The 1 S And 2 S Orbitals To Achieve A 1 S2 2 S2 Electron Configuration:
For each of the following pairs of compounds, state which would have the higher boiling point. Web electron configuration for co, co2+, and co3+ (cobalt and cobalt ions) to write the configuration for the cobalt ions, first we need to write the electron configuration for just cobalt (co). Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. This problem has been solved!