Draw The Alcohol Needed To Form Isobutyl Benzoate
Draw The Alcohol Needed To Form Isobutyl Benzoate - To form isobutyl benzoate you need to react isobutanol with benzoic acid in a reaction called esterification of carboxylic acids with alcohols, in which water is also formed. The general formula for an ester is shown below. Web draw the ester that is formed from the reaction of benzoic acid and ethanol. A chemical reaction between an alcohol and an acid, in which an ester is formed. Isobutyl benzoate is an ester and so an carboxylic acid and an alcohol are needed to form isobutyl benzoate.
The reaction can be written as follows: We need an alcohol to form an ester (isobutyl benzoate). Draw ethanol, which is the alcohol needed to form the ester that smells like grape. Draw the structure of a. This substance is the reference (100 level) for octane ratings. 3) draw balanced equations for the reactions that took place in each of your 6 test tubes from today’s lab. Web expert answer 100% (3 ratings) transcribed image text:
Isobutyl alcohol solution CRM LABSTANDARD
Web this problem has been solved! The ester is formed by the reaction of an alcohol with a carboxylic acid. Isobutyl benzoate is a benzoate ester which is obtained by the condensation of benzoic acid and… q: It is functionally related to a benzoic acid and an isobutanol. If an acid chloride undergoes an addition.
Which of the following alkenes when passed through conc. H2SO4 followed
Isobutyl benzoate is an ester and so an carboxylic acid and an alcohol are needed to form isobutyl benzoate. The general formula for an ester is shown below. So if you start with the carboxylic acid, and you add an alcohol, and a source of protons, you're gonna form your ester, and you're also going.
[Solved] Draw the alcohol needed to form isobutyl benzoate (2
B) write the names of the carboxylic acid and alcohol underneath your drawings. A double sugar molecule made of two monosaccharides bonded together through dehydration synthesis. Isobutyl benzoate is an ester and so an carboxylic acid and an alcohol are needed to form isobutyl benzoate. The ester is formed by the reaction of an alcohol.
Isobutyl benzoate SIELC
Web an ester is an organic compound that is a derivative of a carboxylic acid in which the hydrogen atom of the hydroxyl group has been replaced with an alkyl group. So if you start with the carboxylic acid, and you add an alcohol, and a source of protons, you're gonna form your ester, and.
Solved Draw The Water That Is Formed From The Reaction Of...
Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. Draw ethanol, which is the alcohol needed to form the ester that smells like grape. Here’s the best way to solve it. The catalyst is usually concentrated sulphuric acid. So if you start with the carboxylic.
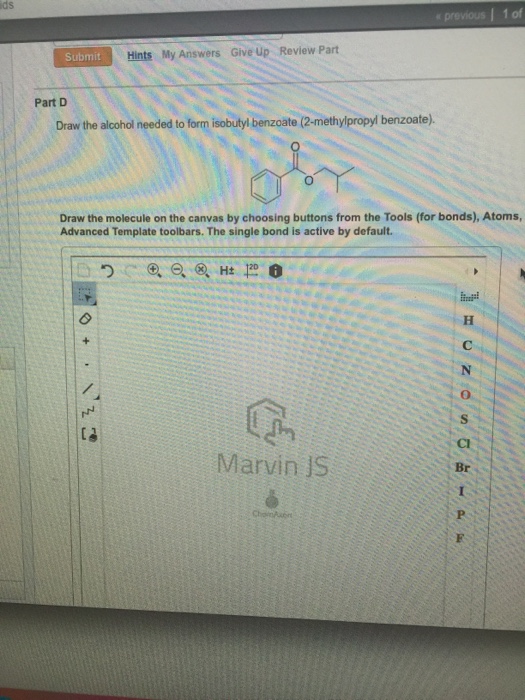
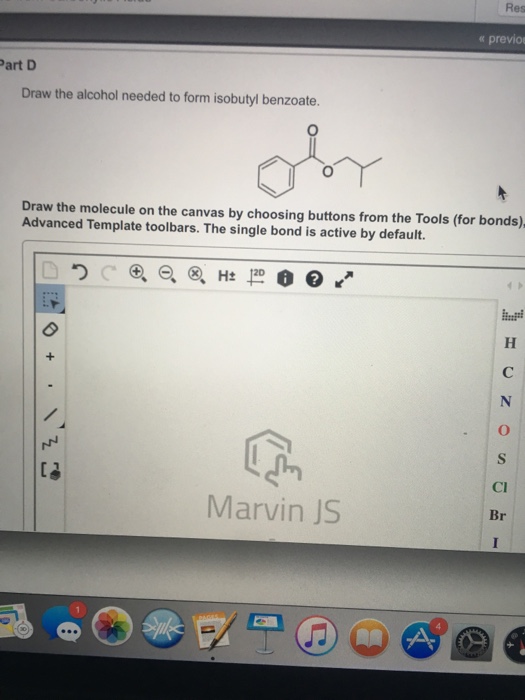
Solved Draw the alcohol needed to form isobutyl benzoate.
So if you start with the carboxylic acid, and you add an alcohol, and a source of protons, you're gonna form your ester, and you're also going to make water in this process. A double sugar molecule made of two monosaccharides bonded together through dehydration synthesis. Web 178.23 g/mol computed by pubchem 2.2 (pubchem release.
What is the difference between isobutyl and sec butyl?
Dry hydrogen chloride gas is used in some cases, but these tend to involve aromatic esters (ones containing a benzene ring). We need an alcohol to form an ester (isobutyl benzoate). The reaction can be written as follows: Constants periodic table draw the alcohol needed to form isobutyl benzoate. Web expert answer 100% (3 ratings).
draw the alcohol needed to form isobutyl benzoate
Draw the structure of a. We don’t have your requested question, but here is a suggested video that might help. So if you start with the carboxylic acid, and you add an alcohol, and a source of protons, you're gonna form your ester, and you're also going to make water in this process. A chemical.
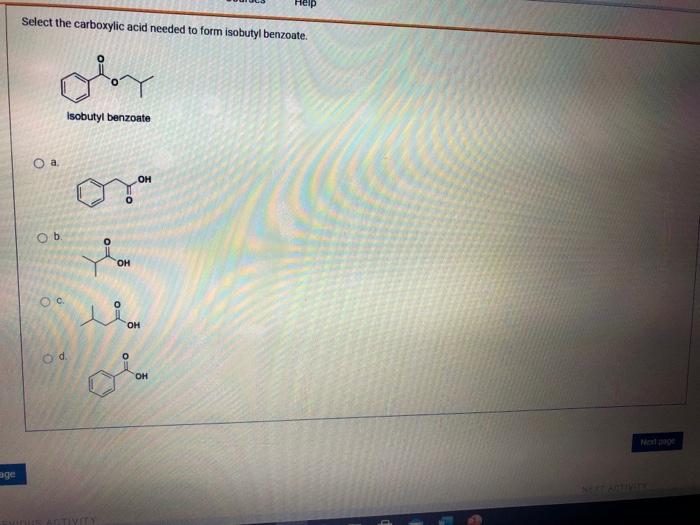
Solved Select the carboxylic acid needed to form isobutyl
The single bond is active. So if you start with the carboxylic acid, and you add an alcohol, and a source of protons, you're gonna form your ester, and you're also going to make water in this process. The general formula for an ester is shown below. Web esters are produced when carboxylic acids are.
draw the alcohol needed to form isobutyl benzoate
To understand how esters are formed from carboxylic acids and alcohols when carboxylic acids and alcohols are heated in the presence of an acid catalyst, they will react to form an ester and water. Web this problem has been solved! Draw ethanol, which is the alcohol needed to form the ester that smells like grape..
Draw The Alcohol Needed To Form Isobutyl Benzoate One way to make an ester is to use a fischer esterification reaction. It is functionally related to a benzoic acid and an isobutanol. The single bond is active. Web 178.23 g/mol computed by pubchem 2.2 (pubchem release 2021.10.14) dates create: If an acid chloride undergoes an addition reaction with an organometallic reagent, what type of…
Web 178.23 G/Mol Computed By Pubchem 2.2 (Pubchem Release 2021.10.14) Dates Create:
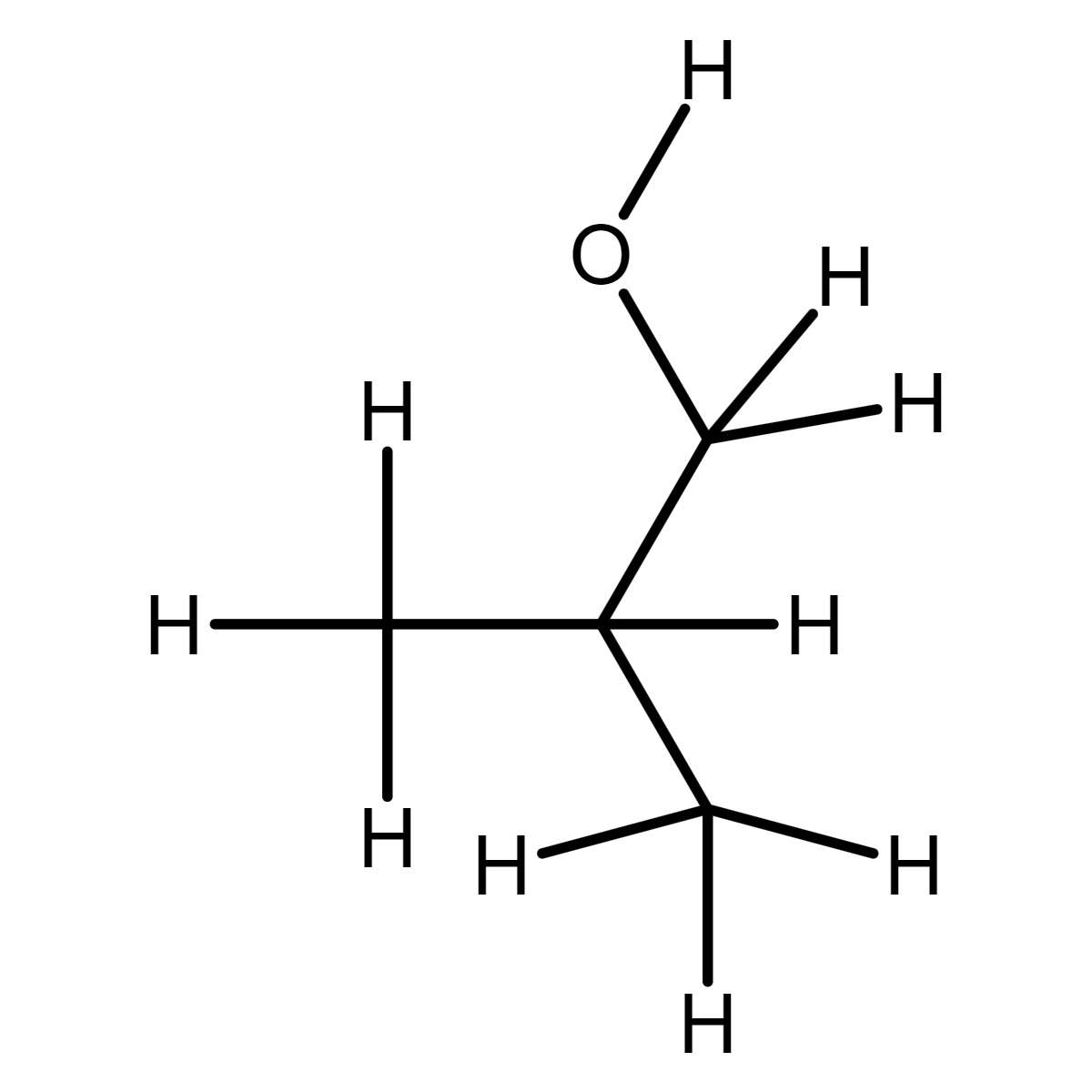
The alcohol needed is the isobutanol. Web chemistry chemistry questions and answers draw the alcohol needed to form isobutyl benzoate. A chemical reaction between an alcohol and an acid, in which an ester is formed. A double sugar molecule made of two monosaccharides bonded together through dehydration synthesis.
Isobutyl Benzoate Is An Ester And So An Carboxylic Acid And An Alcohol Are Needed To Form Isobutyl Benzoate.
The single bond is active by default. Web this problem has been solved! Web draw the ester that is formed from the reaction of benzoic acid and ethanol. Identify the following as a fatty acid, soap, triacylglycerol, wax, glycerophospholipid, sphingolipid, or steroid:
Isobutyl Benzoate Is A Benzoate Ester Which Is Obtained By The Condensation Of Benzoic Acid And… Q:
One way to make an ester is to use a fischer esterification reaction. In nitration of methyl benzoate why do you pour the reaction mixture onto ice chunks? View available hint (s) oh. We need an alcohol to form an ester (isobutyl benzoate).
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. The reaction between isobutanol and benzoic acid will produce isobutyl benzoate, with water as a byproduct. To understand how esters are formed from carboxylic acids and alcohols when carboxylic acids and alcohols are heated in the presence of an acid catalyst, they will react to form an ester and water. Draw ethanol, which is the alcohol needed to form the ester that smells like grape.