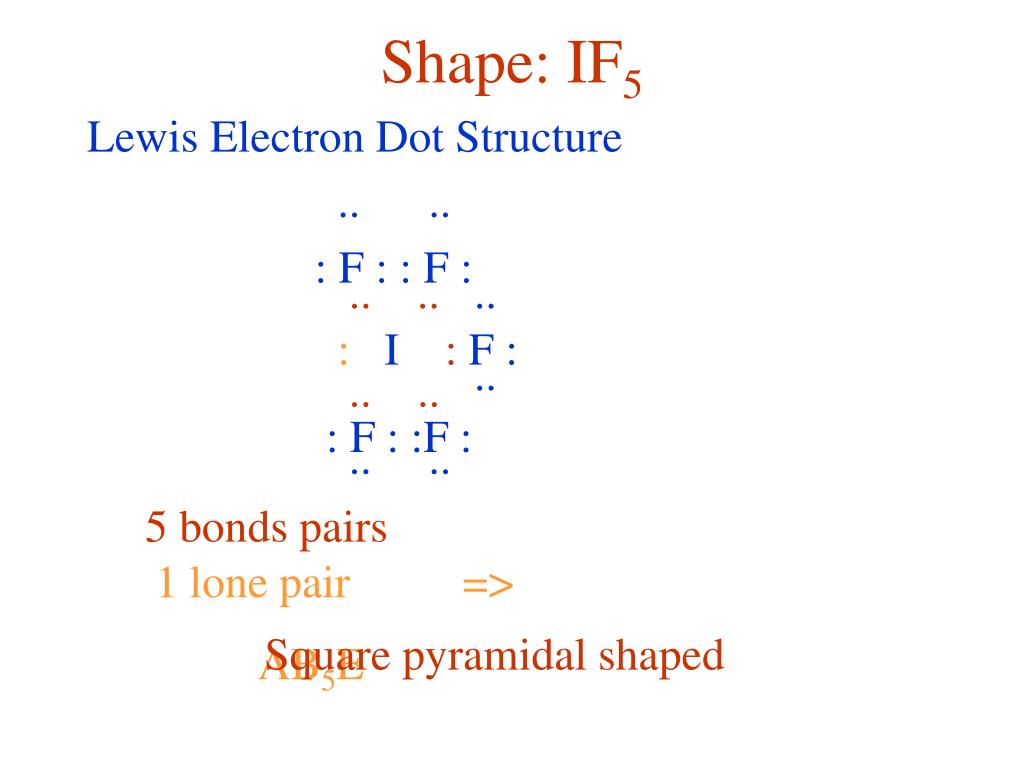
Draw An Appropriate Lewis Structure For If5
Draw An Appropriate Lewis Structure For If5 - #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. The first step is to count all the valence electrons of each molecule. If5 lewis dot structure by counting valence electrons on the iodine atom. Web drawing lewis structures for molecules with one central atom: Web 0:00 / 1:40 lewis dot structure of if5 (iodine pentafluoride) kentchemistry.com 24.9k subscribers 48k views 12 years ago every video i quickly take you through how to draw the lewis.
Web steps of drawing if5 lewis structure step 1: If5 lewis dot structure by counting valence electrons on the iodine atom. Web science chemistry chemistry questions and answers draw (on paper) a lewis structure for if_5 and answer the following questions based on your drawing. Web the lewis structure (lewis dot diagram) for if5. In order to find the total valence electrons in a if5 (iodine. See the big list of lewis structures. #1 draw a rough sketch of the structure first, determine the total number of valence electrons
If5 Lewis Structure
For the if5 lewis structure, calculate the total number of valence electrons for the if5 molecule. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web to sketch the if5 lewis structure by following these instructions: While selecting the atom, always put the least electronegative atom at the center..
IF5 Molecular Geometry, Bond Angles and Electron Geometry YouTube
Web a video explanation of how to draw the lewis dot structure for iodine pentafluoride, along with information about the compound including formal charges, pola. Web 10k views 3 years ago lewis structures. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. 2).
IF5 Molecular Geometry Science Education and Tutorials
For selecting the center atom, you have to remember that the atom which is less. Web 5 steps to draw the lewis structure of if5 step #1: #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down.
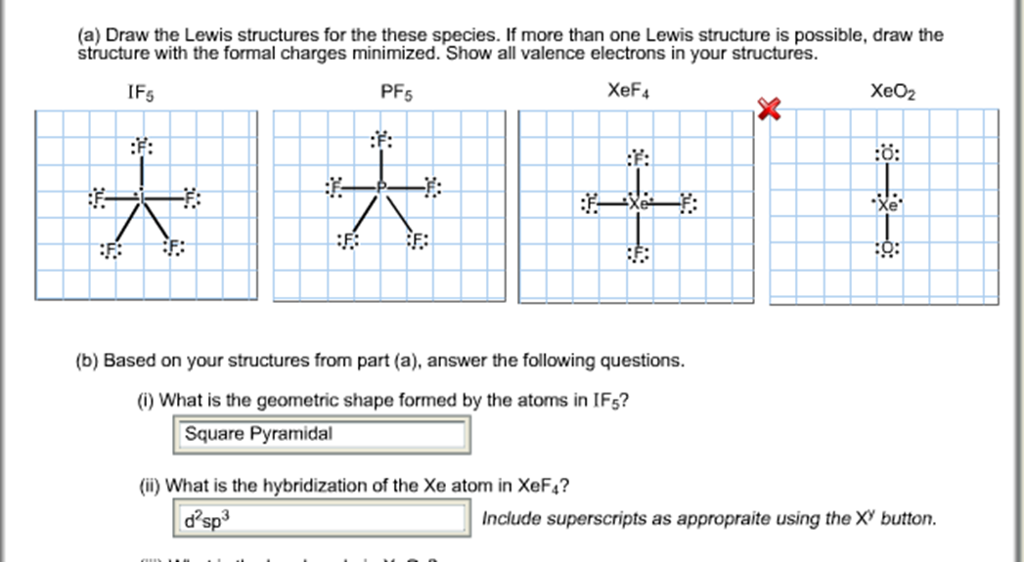
Draw the Lewis structure of iodine pentafluoride, IF5.
For each compound draw an appropriate lewis structure, determine the molecular geometry using vsepr theory, determine whether the molecule is polar and identify the hybridization of all interior atoms: Find the total valence electrons in if5 molecule. For the central iodine atom: 3) describe the bonding in the following molecules bonding molecule. Web 10k views.
IF5 Lewis Structure How to Draw the Lewis Structure for IF5 YouTube
In order to find the total valence electrons in a if5 (iodine. The central iodine atom a. For selecting the center atom, you have to remember that the atom which is less. Fill outer atoms with electrons 5. #1 draw a rough sketch of the structure first, determine the total number of valence electrons This.
If5 Lewis Structure
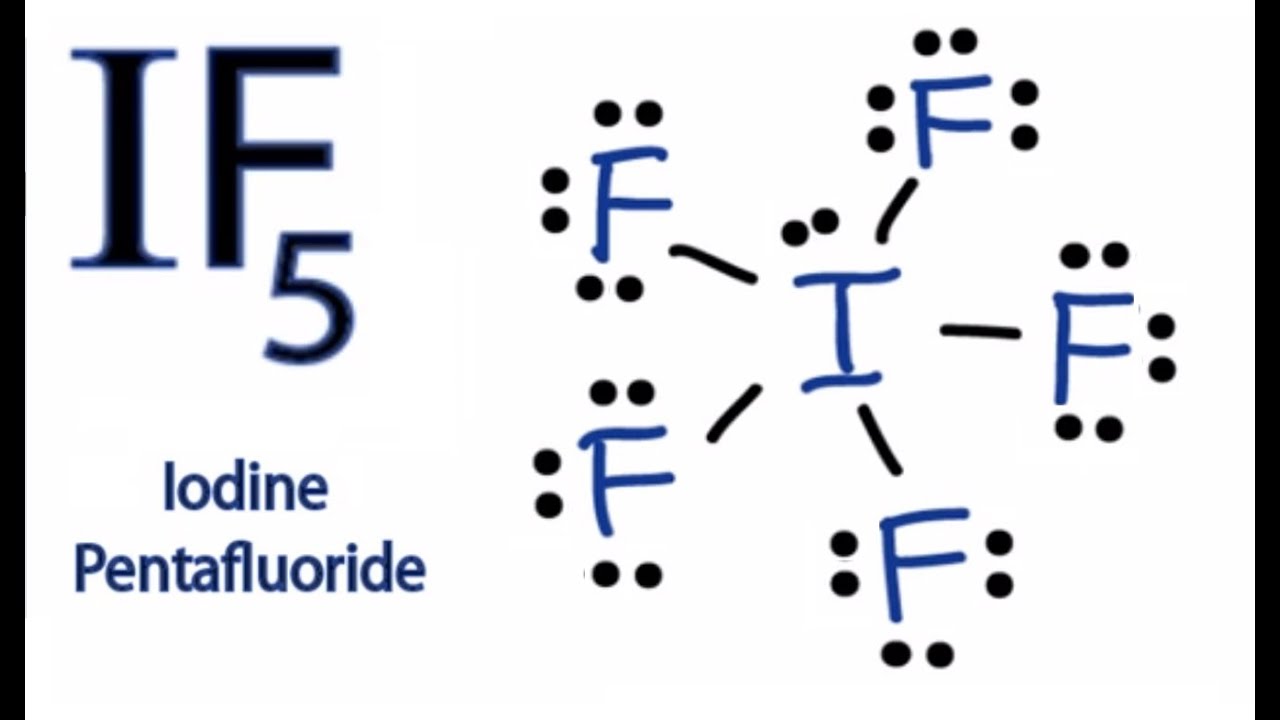
Determine the total number of valence (outer shell) electrons. Web 1) draw a lewis structure for iof5 and calculate the formal charges on each atom. Remember that iodine (i) can hold more than eight valence electrons. Draw the molecule by placing atoms on the grid and connecting them with bonds. If5 lewis dot structure by.
If5 Lewis Structure
Iodine is the least electronegative. Valance electron determination considering the if5 lewis structure, both iodine and fluorine atom contain 7 valence electrons. Which has the larger bond angle? While selecting the atom, always put the least electronegative atom at the center. Web steps by using the following steps, you can easily draw the lewis structure.
IF5 Lewis, VSEPR and Box diagram for hybridiaztion YouTube
Web 10k views 3 years ago lewis structures. Which has the larger bond angle? Web 5 steps to draw the lewis structure of if5 step #1: For each compound draw an appropriate lewis structure, determine the molecular geometry using vsepr theory, determine whether the molecule is polar and identify the hybridization of all interior atoms:.
If5 Lewis Structure
Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Find more chemistry widgets in wolfram|alpha. There are a total of 42 valence electrons. Web to sketch the if5 lewis structure by following these instructions: For if5, we have a total of 42 valence electrons. Web science chemistry chemistry questions.
If5 Lewis Structure
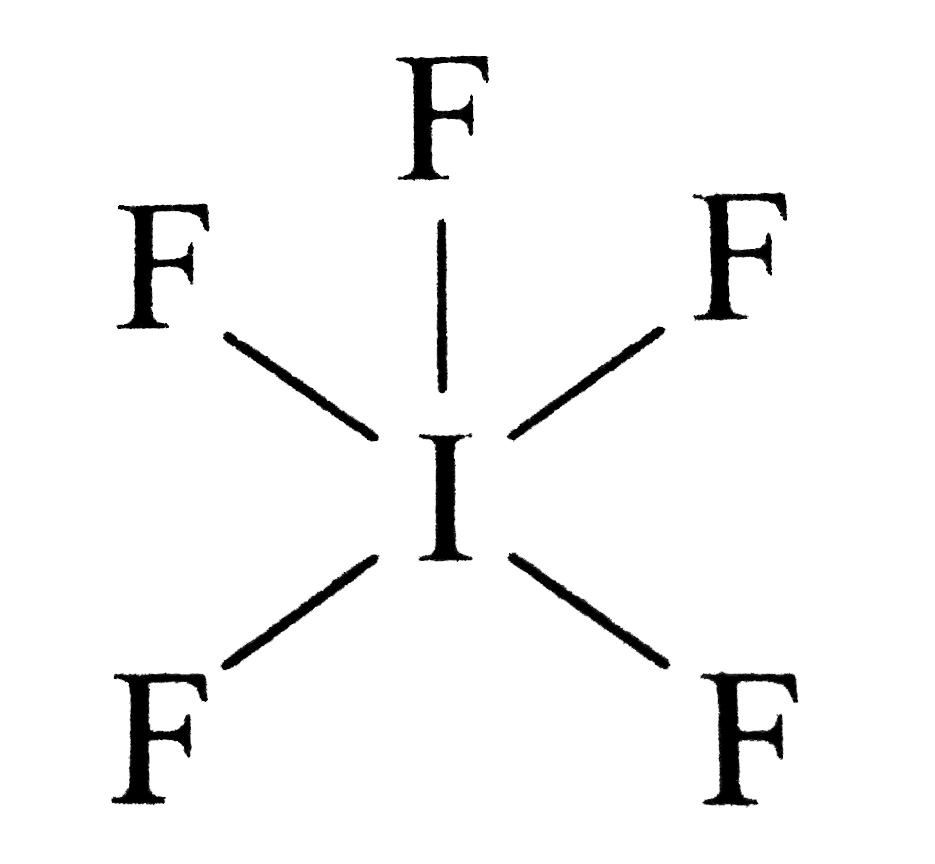
Web steps to properly draw the if 5 lewis structure, follow these steps: This gives it ax5e1 shape by vsepr, and. The central iodine atom a. Determine the total number of valence (outer shell) electrons. Web science chemistry chemistry questions and answers draw the lewis dot structure of the molecule if5 and determine the electron.
Draw An Appropriate Lewis Structure For If5 In the case of if5, the iodine atom has 7. Iodine is the least electronegative. Web the lewis structure (lewis dot diagram) for if5. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. While selecting the atom, always put the least electronegative atom at the center.
Iodine Is Below Period Two On The Periodic Table So It Can Have.
Web in the lewis structure for if5 you'll need to put a total of 12 valence electrons on the iodine atom in order to draw the lewis structure. For the if5 lewis structure, calculate the total number of valence electrons for the if5 molecule. Web 0:00 / 1:40 lewis dot structure of if5 (iodine pentafluoride) kentchemistry.com 24.9k subscribers 48k views 12 years ago every video i quickly take you through how to draw the lewis. Web science chemistry chemistry questions and answers draw the lewis dot structure of the molecule if5 and determine the electron and molecular geometries around the i atom.
3) Describe The Bonding In The Following Molecules Bonding Molecule.
This gives it ax5e1 shape by vsepr, and. Web hello, it's time for your daily chemistry dose! Let us follow a few steps. Put one electron pair in each bond 4.
While Selecting The Atom, Always Put The Least Electronegative Atom At The Center.
#1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. For each compound draw an appropriate lewis structure, determine the molecular geometry using vsepr theory, determine whether the molecule is polar and identify the hybridization of all interior atoms: Iodine is the least electronegative. Find more chemistry widgets in wolfram|alpha.
Calculate The Total Number Of Valence Electrons.
The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web steps by using the following steps, you can easily draw the lewis structure of if 5: Here, the given molecule is if5 (iodine pentafluoride). In order to find the total valence electrons in a if5 (iodine.