Draw All Resonance Structures For The Carbonate Ion Co32-
Draw All Resonance Structures For The Carbonate Ion Co32- - It has a total of 24. (b) what is molecular and electron geometry of carbonate ion? If there are equivalent resonance structures, draw all of them. Web the carbonate ion is an example of a molecule for which we can draw three equivalent structures. Identify which orbitals overlap to create each bond.openstax™ is a registered trademark,.
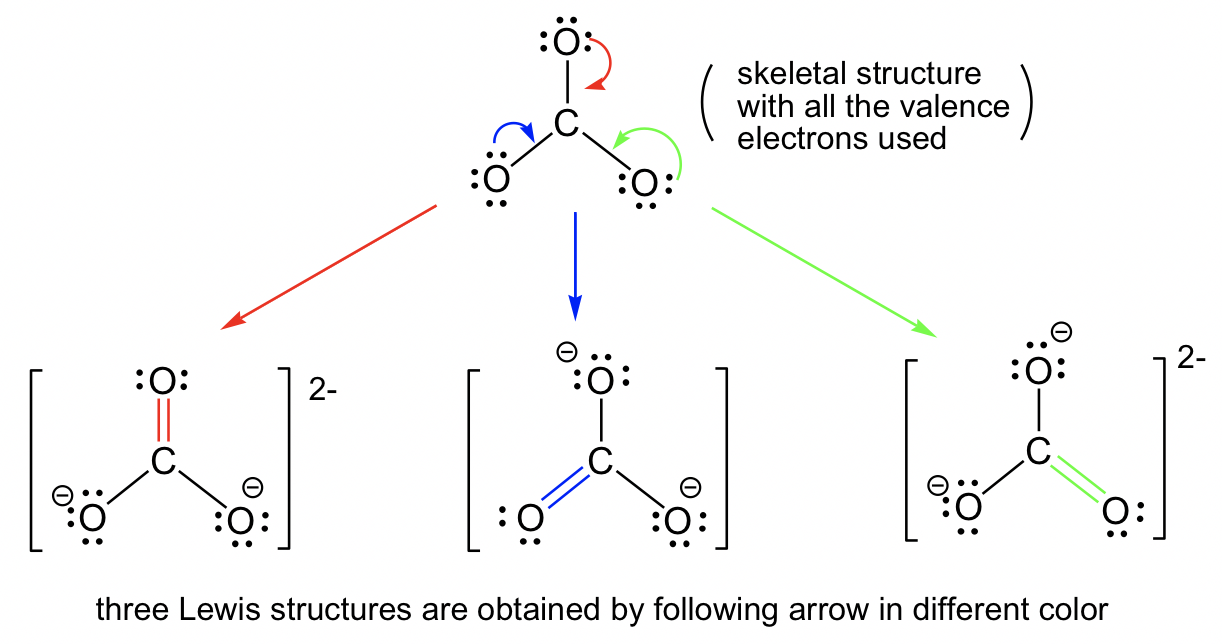
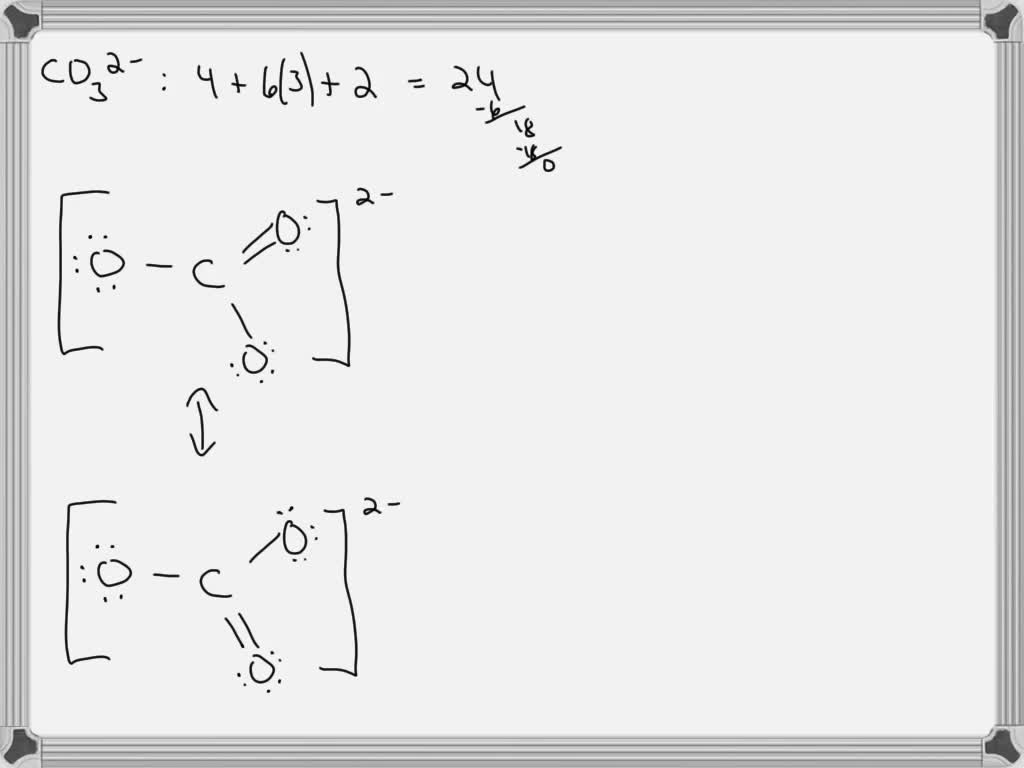
It has a total of 24. Web draw three resonance structures for carbonate ion, co32, and assign formal charges on all the atoms. Identify which orbitals overlap to create each bond.openstax™ is a registered trademark,. Determine the formal charge of each atom. Draw the lewis structure of ozone (o3) showing all possible resonance structures if there are any. Web the lewis structure of carbonate ion can be drawn as follows: The three oxygens are drawn in the shape of a triangle with the carbon at the center of the triangle.
1.3 Resonance Structures Organic Chemistry I
Web for the carbonate ion, co 3 2−, co 3 2−, draw all of the resonance structures. For the carbonate ion, co3 2−, draw all of the resonance structures. Web answer link the answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. Remember to indicate.
SOLVED For CO32 , carbonate ion, draw the Lewis structure (by
(b) what is molecular and electron geometry of carbonate ion? Web answer link the answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. (a) draw all of them. Web draw the resonance structures of molecules or ions that exhibit delocalization. We start with a valid.
Write the resonance structures of CO3^2 and HCO3^
A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, h 3. This problem has been solved! Discusses resonance and bond order. Determine the relative stability of resonance structures using a set of rules. You'll get a detailed solution from a subject matter expert that helps you learn core..
How To Draw The Lewis Structure of CO3 2 (Carbonate Ion) Chemistry
(a) draw all of them. H c 0 s f this problem has been solved! This video discusses the resonance structure of this polyatomic ion as well as the. Web how to draw resonance structure of carbonate ion as with ozone, the carbonate ion’s electronic structure cannot be explained by a single lewis electron structure..
How to draw the Lewis structure of CO3 2 (Carbonate ion) YouTube
(b) what is molecular and electron geometry of carbonate ion? Web the lewis structure of carbonate ion can be drawn as follows: Web draw the resonance structures of molecules or ions that exhibit delocalization. Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are neutral. Determine the.
Problem 4.3Explain the structure of CO3^2 (carbonate) ion in termsof
If there are equivalent resonance structures, draw all of them. This video discusses the resonance structure of this polyatomic ion as well as the. Determine the relative stability of resonance structures using a set of rules. Draw the lewis structure of ozone (o3) showing all possible resonance structures if there are any. Identify which orbitals.
CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2
(b) what is molecular and electron geometry of carbonate ion? Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are neutral. Web answer link the answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. Do not include.
CO32 Molecular Geometry, Shape and Bond Angles (Carbonate Ion) YouTube
Web how to draw resonance structure of carbonate ion as with ozone, the carbonate ion’s electronic structure cannot be explained by a single lewis electron structure. Determine the formal charge of each atom. Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are neutral. This video discusses.
Lewis Structure of the Carbonate Ion (CO32) YouTube
The three oxygens are drawn in the shape of a triangle with the carbon at the center of the triangle. Use the concept of resonance to explain structural features of molecules and ions. Discusses resonance and bond order. Each structure has one double bond and two single bonds, suggesting that one of the bonds is.
Solved Draw All Resonance Structures For The Carbonate Io...
Web the carbonate ion is an example of a molecule for which we can draw three equivalent structures. The three oxygens are drawn in the shape of a triangle with the carbon at the center of the triangle. Draw the lewis structure of carbonate ion (co32) showing all possible resonance structures if there are any..
Draw All Resonance Structures For The Carbonate Ion Co32- Because carbon is the least electronegative element, we place it in the central position: Expert answer step 1 resonance: Remember to indicate formal charges on atoms that have them in each structure. Do not include overall ion charges or formal charges in your drawing. Web unlike o 3, though, the actual structure of co 32− is an average of three resonance structures.
H C 0 S F This Problem Has Been Solved!
Because carbon is the least electronegative element, we place it in the central position: You do not need to include resonance arrows. It has a total of 24. This problem has been solved!
(B) What Is Molecular And Electron Geometry Of Carbonate Ion?
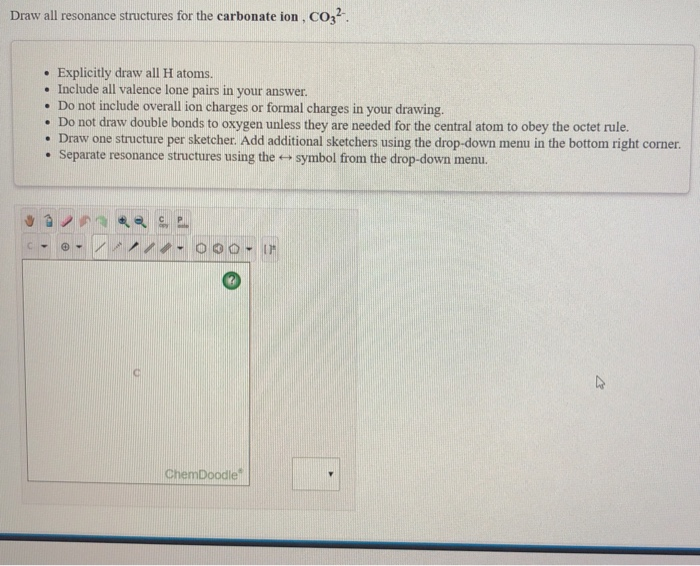
Draw one structure per sketcher box, and separate added sketcher boxes with the ↔ symbol. The correct lewis structure for this ion has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. Do not include overall ion charges or formal charges in your drawing. (a) draw all of them.
(B) What Is Molecular And Electron Geometry Of Carbonate Ion?
Web draw three resonance structures for carbonate ion, co32, and assign formal charges on all the atoms. Identify which orbitals overlap to create each bond.openstax™ is a registered trademark,. Draw the lewis structure of ozone (o3) showing all possible resonance structures if there are any. Discusses resonance and bond order.
Each Of The Singly Bonded Oxygen Atoms Bears A Formal Charge Of ‐1 And All Other Atoms Are Neutral.
Expert answer step 1 resonance: A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, h 3. Web draw the resonance structures of molecules or ions that exhibit delocalization. Identify the resonance contributing atoms.