Draw A Sodium Atom
Draw A Sodium Atom - Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web • the atomic mass of sodium is 22.989. Sodium is the 11th element of the periodic table so its atomic number is 11. #1 write protons, neutrons, and electrons of sodium atom #2 draw nucleus of sodium atom #3 draw 1 st electron shell #4 draw 2 nd electron shell #5 draw 3 rd electron shell let’s break down each step in detail.
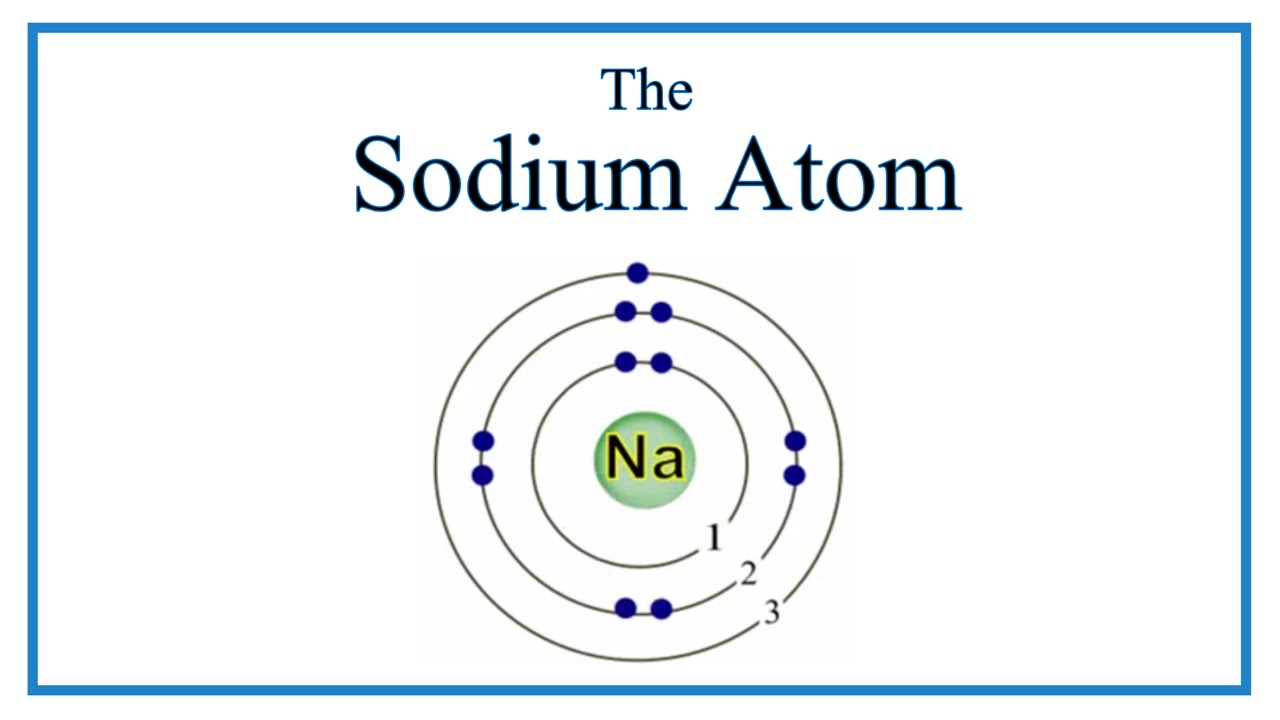
Additionally, sodium is found in group one, which means it has one valence electron. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Its outer shell will then have no electrons. Web updated on november 05, 2019. Web sodium is a chemical element; In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. 1 s 2 2 s 2 2 p 6 3 s 1 the valence shell of the sodium atom is 3 3.
Diagram representation of the element sodium Vector Image
Similarly, neon has a complete outer 2n shell containing eight electrons. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Web what happens when a sodium atom becomes a sodium ion? It is as though the outer shell has vanished. Web sodium is atomic number.
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium
Its outer shell will then have no electrons. Web this video shows how to draw the orbital diagram of sodium (na). Additionally, sodium is found in group one, which means it has one valence electron. Web sodium is atomic number 11, and it is a metal, as it lies to the left of the dotted.
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium
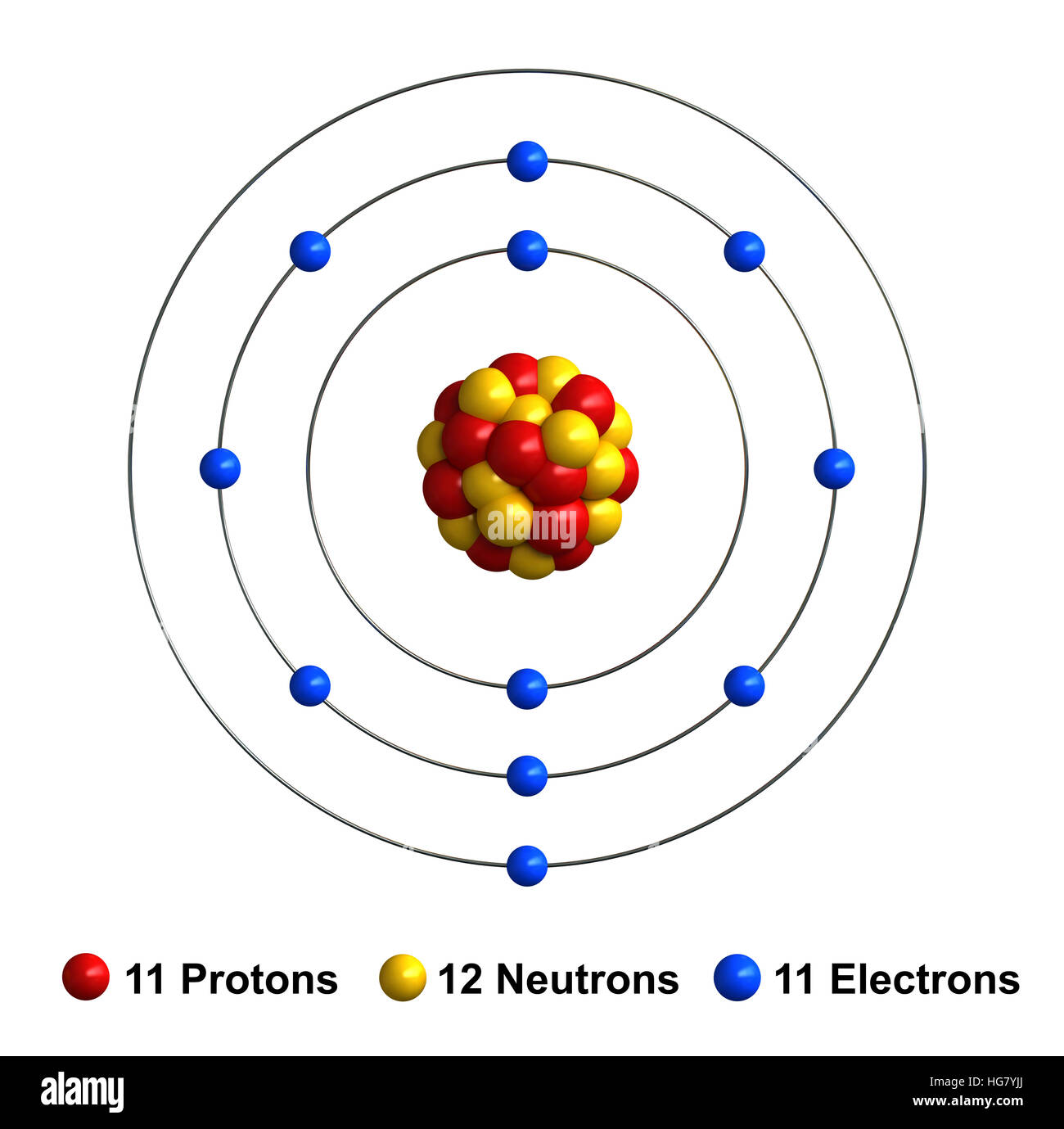
It has a total of 11 11 protons and 11 11 electrons. I show you where sodium is on the periodic table and how to determine how. Additionally, sodium is found in group one, which means it has one valence electron. Web this video shows how to draw the orbital diagram of sodium (na). Example.
FileElectron shell 011 sodium.png Wikimedia Commons
Even a minute amount of water can create this type of reaction. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Sodium has 2 electrons in.
Sodium atom polizworth
Atomic number of sodium is 11. Web updated on november 05, 2019. The atomic number of an element is equal to the number of protons and electrons in that element. Web that is, the number of electrons and protons in the sodium atom is eleven. Example \(\pageindex{2}\) draw the bohr's model for sodium (na). To.
Atom sodium Royalty Free Vector Image VectorStock
Its outer shell will then have no electrons. It has a total of 11 11 protons and 11 11 electrons. Web for atoms with more than two protons and two electrons, a few rules need to be introduced to diagram them in a way that represents their chemical properties. Thus, the sodium ion has a.
3d render of atom structure of sodium isolated over white background
Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. #1 write protons, neutrons, and electrons of sodium atom I show you where sodium is on the periodic table and how to determine how. Here are electron shell atom diagrams for the elements, ordered by increasing.
Atomic Structure of the Sodium Atom (Na) YouTube
Web the sodium orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, and the remaining one electron in the 3s orbital. Even a minute amount of water can create this type of reaction. It is as though the outer shell has vanished..
Sodium Na (Element 11) of Periodic Table NewtonDesk
It is in group 1 of the periodic table. Even a minute amount of water can create this type of reaction. #1 write protons, neutrons, and electrons of sodium atom For that, we have electron shell diagrams. Web that is, the number of electrons and protons in the sodium atom is eleven. Sodium usually forms.
Sodium Table of Elements by Shrenil Sharma
Web what happens when a sodium atom becomes a sodium ion? It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. A sodium atom has 1 electron in its outer shell. Web steps here’s how you can draw the bohr model of sodium step by step. The electronic.
Draw A Sodium Atom Sodium is an atom in the periodic table with atomic number 11 11 and mass number 23 23. Web sodium is atomic number 11, and it is a metal, as it lies to the left of the dotted zigzag line. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. It is in group 1 of the periodic table. Web updated on november 05, 2019.
Web This Video Shows How To Draw The Orbital Diagram Of Sodium (Na).
Its outer shell will then have no electrons. Web sodium is atomic number 11, and it is a metal, as it lies to the left of the dotted zigzag line. Web for atoms with more than two protons and two electrons, a few rules need to be introduced to diagram them in a way that represents their chemical properties. Sodium usually forms ionic compounds involving the na+ cation.
Web Updated On November 05, 2019.
Even a minute amount of water can create this type of reaction. Web steps here’s how you can draw the bohr model of sodium step by step. A bhor's model can be used to diagram the location of electrons in each energy shell for an atom. Web • the atomic mass of sodium is 22.989.
Thus, The Sodium Ion Has A Net Charge Of 1+, And It Has Become A Cation—A Positively Charged Ion.
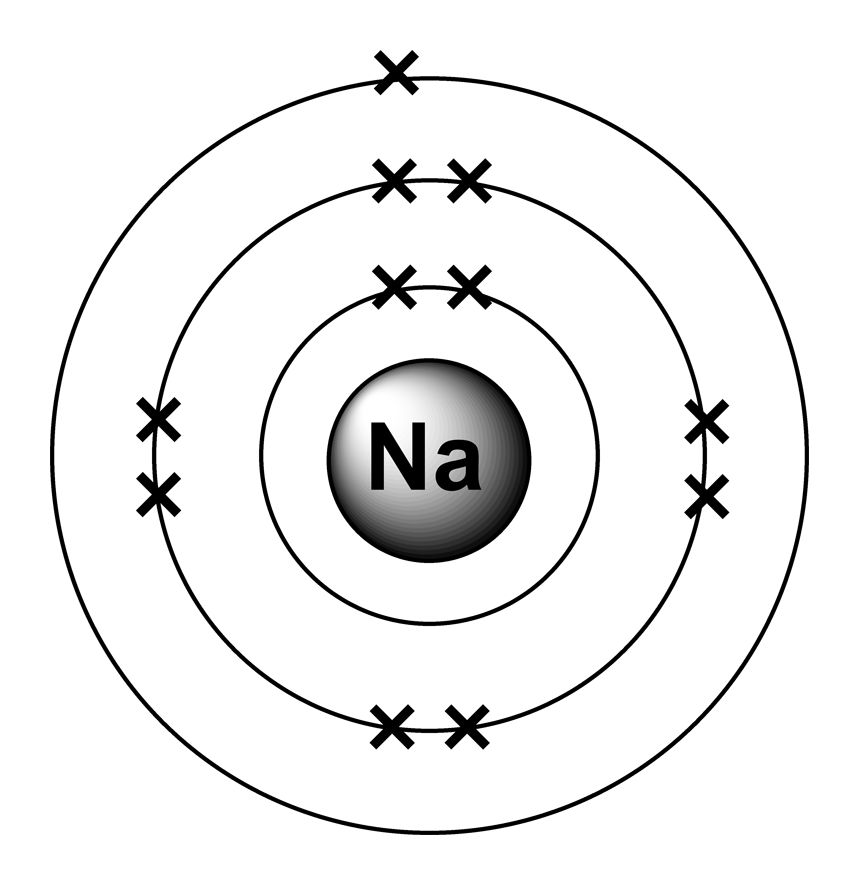
Web that is, the number of electrons and protons in the sodium atom is eleven. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Web the sodium atom (na) is commonly used for examples and practice problems in chemistry. To illustrate this using an electron dot diagram, we would.
I Show You Where Sodium Is On The Periodic Table And How To Determine How.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web what happens when a sodium atom becomes a sodium ion? It is as though the outer shell has vanished. Web sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon.