Draw A Nitrogen Atom
Draw A Nitrogen Atom - So three bonds and one lone pair of electrons, the formal charge is equal to zero. #1 write protons, neutrons, and electrons of nitrogen atom #2 draw nucleus of nitrogen atom #3 draw 1 st electron shell #4 draw 2 nd electron shell. This problem has been solved! Draw resonance structures of some molecules. As we can see, all the atoms inside the nf3 molecule have the least possible formal charge values.
[a] nitrogen’s protons and neutrons go into the nucleus. So we have a pattern. Each carbon atom has four bonds. Web write lewis symbols for neutral atoms and ions. Each hydrogen atom has one bond. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. These three electrons have unpaired spins.
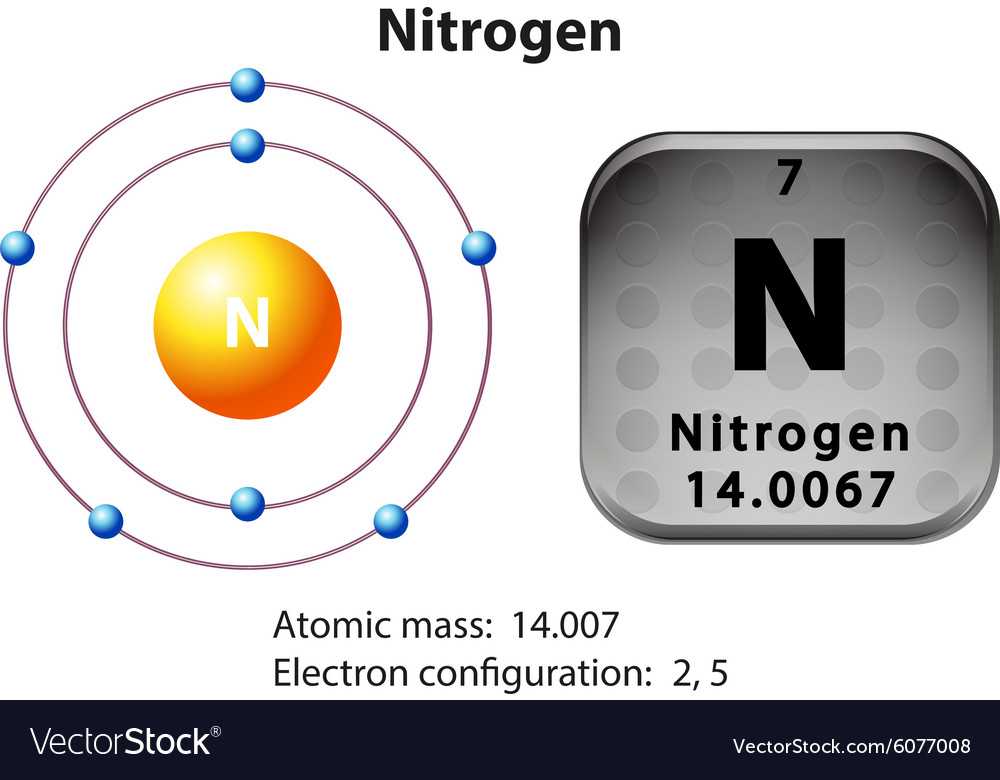
Symbol and electron diagram for nitrogen Vector Image
Web here’s how you can draw the bohr model of nitrogen step by step. We’ll use a bohr diagram to visually represent where the electrons. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. Let’s draw and understand this lewis dot structure step by step. After.
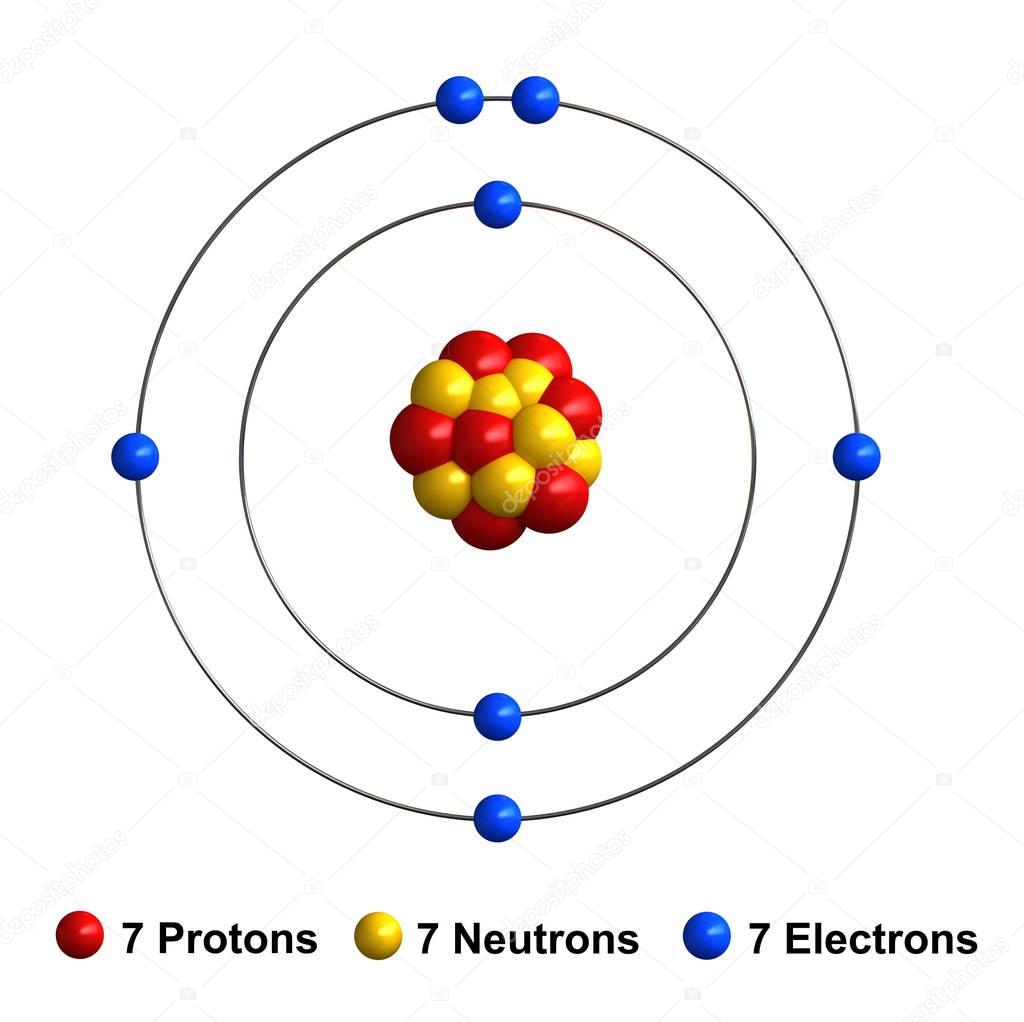
3d render of atom structure of nitrogen — Stock Photo © oorka5 136542114
Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Here, we will draw the bohr diagram of the..
Diagram representation of the element nitrogen Vector Image
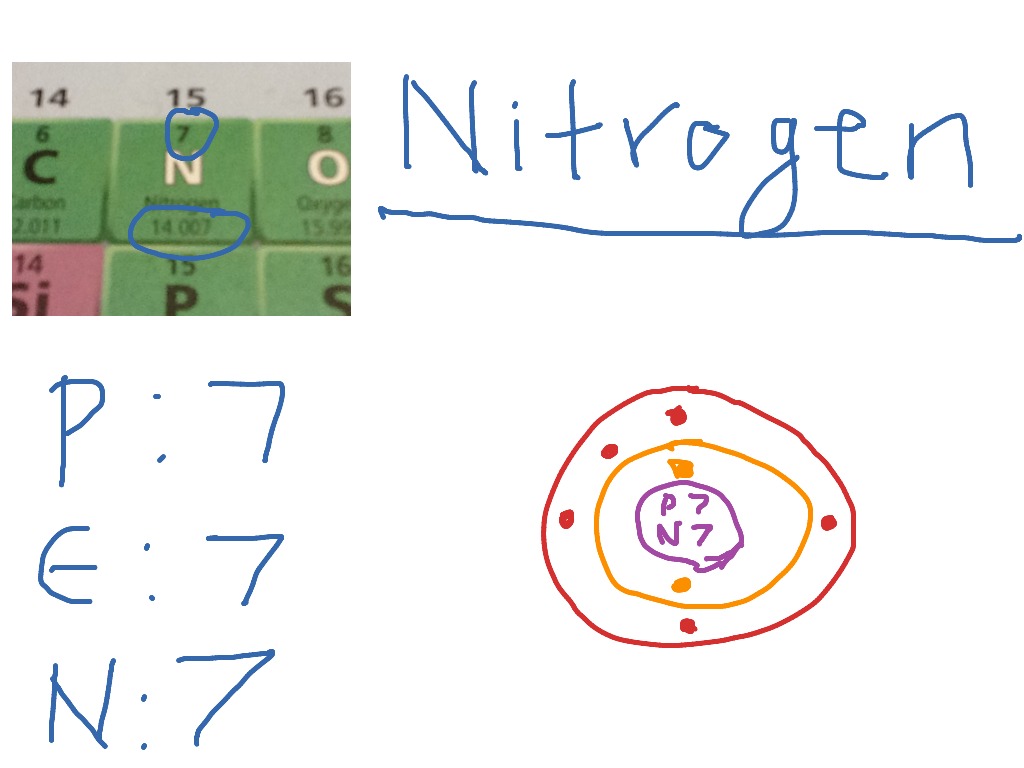
The following image attached can explain this more clearly, we can see after drawing the sketch, remaining electrons are given around the atoms. The nitrogen atom (n) is at the center and it is surrounded by 3 oxygen atoms (o). Draw a small circle, inside that circle, write p = 7 and n = 7.
Bohr model Description & Development Britannica
Draw resonance structures of some molecules. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. There are four electron groups around nitrogen, three bonding pairs and one lone pair. Web bohr diagram is very interesting and easy to draw. As we can.
How to draw a Nitrogen Atom Science ShowMe
Web the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (figure \(\pageindex{1}\)). This problem has been solved! The nitrogen atom (n) is at the center and it is surrounded by 3 oxygen atoms (o). Electrons are the same.
Nitrogen Structure
Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that..
Nitrogen Facts, Symbol, Discovery, Properties, Uses
Draw resonance structures of some molecules. Each nitrogen atom has three bonds. These three electrons have unpaired spins. As we can see, all the atoms inside the nf3 molecule have the least possible formal charge values. [a] nitrogen’s protons and neutrons go into the nucleus. Here, we will draw the bohr diagram of the. In.
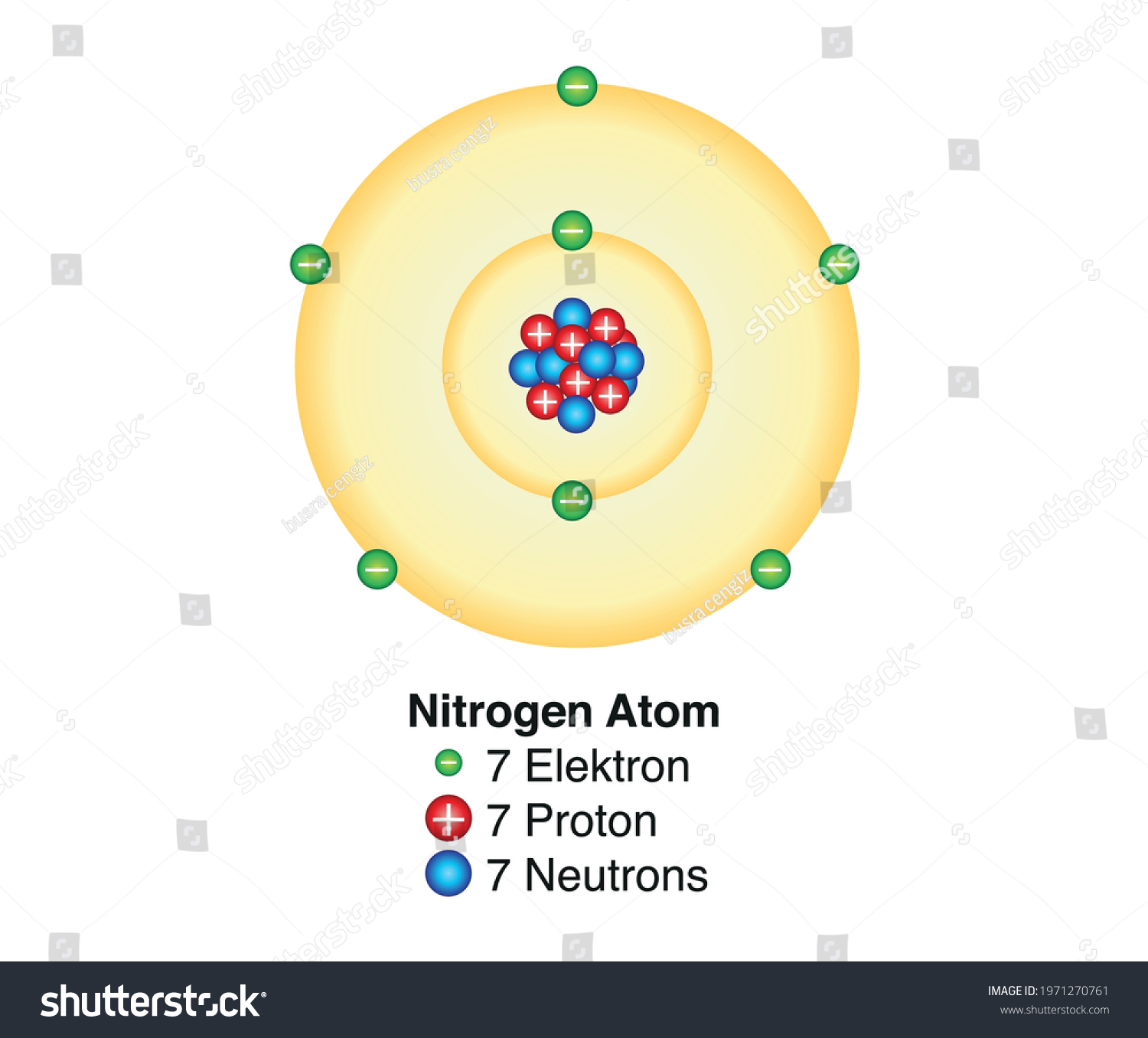
Basic Model Nitrogen Atom Containing Protons Stock Vector (Royalty Free
Web in this video we'll look at the atomic structure and bohr model for the nitrogen atom (n). Web in this case, nitrogen with the highest bonding sites is the central atom. Web write lewis symbols for neutral atoms and ions. Let’s draw and understand this lewis dot structure step by step. Web we found.
Nitrogen Wikipedia
Assess the stability of a structure by considering formal charges of atoms. Web write lewis symbols for neutral atoms and ions. Each hydrogen atom has one bond. The next five electrons go into the second energy level. Each nitrogen atom has three bonds. Web here’s how you can draw the bohr model of nitrogen step.
Nitrogen atom heavykesil
I show you where nitrogen is on the periodic table and how to determine how many valence electrons it has. Let’s break down each step in detail. Therefore, we have attained our most perfect lewis structure diagram. Web write lewis symbols for neutral atoms and ions. Draw lewis structures depicting the bonding in simple molecules..
Draw A Nitrogen Atom Assess the stability of a structure by considering formal charges of atoms. Web bohr diagram is very interesting and easy to draw. Draw lewis structures depicting the bonding in simple molecules. Web write lewis symbols for neutral atoms and ions. [a] nitrogen’s protons and neutrons go into the nucleus.
#1 Write Protons, Neutrons, And Electrons Of Nitrogen Atom #2 Draw Nucleus Of Nitrogen Atom #3 Draw 1 St Electron Shell #4 Draw 2 Nd Electron Shell.
We’ll use a bohr diagram to visually represent where the electrons. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Web we found the nitrogen to have a formal charge of zero. Draw lewis structures depicting the bonding in simple molecules.
These Dots Are Arranged To The Right And Left And Above And Below.
Let’s draw and understand this lewis dot structure step by step. The nitrogen atom (n) is at the center and it is surrounded by 3 oxygen atoms (o). Nitrogen will be the central atom draw the lewis diagram as below: Web [q] nitrogen has 7 protons, 7 neutrons, and 7 electrons.
Web Find The Central Atom:
The electronic configuration of nitrogen is 1s^2 2s^2 2p^3 the nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. On a sheet of paper, draw a nitrogen atom, then flip the card to see if you drew it correctly. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that.
Web In Chem 101A, We Will Focus On Drawing Lewis Structures Of Molecules And Polyatomic Ions That Have One Central Atom With Several Other Atoms Attached To It.
The next five electrons go into the second energy level. Draw a small circle, inside that circle, write p = 7 and n = 7 the first outer shell holds 2 electrons so draw, 2 electrons on the circle Draw resonance structures of some molecules. Web nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with hund’s rule.