Draw A Lewis Structure For Nh3
Draw A Lewis Structure For Nh3 - Draw the lewis structure of ammonia (nh3). The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Place the nitrogen atom in the center of the structure and. Drawing the lewis structure for nh3 it is helpful if you: Draw the lewis structure for nh3.
Web to draw the nh3 lewis structure, follow these steps: Web learn the steps to draw the lewis structure of nh3 (ammonia) in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in. + show transcribed image text there are 3 steps to solve this one. Be sure to have the correct number of electrons. Here, the given molecule is nh3 (ammonia). Valence electrons are the electrons in the outermost energy level of an atom. This problem has been solved!
Lewis Dot Diagram Of Nh3
Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Be sure to have the correct number of electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The second step is to add valence electrons to the three.
NH3 Lewis Structure , Valence Electrons ,Formal Charge,Polar or Non
Calculate the total number of valence electrons. Web this problem has been solved! Web to draw the nh3 lewis structure, follow these steps: Web 6 steps to draw the lewis structure of nh3 step #1: It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. Count the.
How to draw NH3 Lewis Structure? Science Education and Tutorials
It is eight to form a single nh3 molecule. Web chemistry chemistry questions and answers draw the lewis structure of ammonia (nh3). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture.
Lewis Structure NH3 plus dipoles, shape, angles and formal charge
For resonance structures there must be a double or triple bond present, which is not the case with nh3. Please include all nonbonding electrons. This chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia. There are 8 valence electrons available for the lewis structure for nh 3. The.
Lewis Dot Diagram Of Nh3
This chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia. It is eight to form a single nh3 molecule. It also is a good example of a molecule with a trigonal prymidal molecular geometry. You'll get a detailed solution from a subject matter expert that helps you learn.
NH3 Molecular Geometry Science Education and Tutorials
This problem has been solved! Web 0:00 / 3:17 lewis structure: Find how many electrons are required in total: For the nh3 structure use the periodic table to find the total number of valence electrons for the nh3. This chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia..
NH3 (ammonia) Lewis dot structure YouTube
Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web chemistry questions and answers. Web 0:00 / 3:17 lewis structure: Web we're going to do the lewis structure for nh3: Determine the total number of valence electrons for all the atoms in nh3, which is 5 (for nitrogen).
How to draw NH3 Lewis Structure? Science Education and Tutorials
On the periodic table, nitrogen is in group 5 or 15 so it has 5 valence electrons, and then hydrogen is in group 1. Find how many electrons are required in total: Web we're going to do the lewis structure for nh3: Web learn the steps to draw the lewis structure of nh3 (ammonia) in.
NH3 Lewis Structure How to Draw the Dot Structure for NH3 YouTube
Draw the lewis structure of ammonia (nh3). Start by counting the total number of valence electrons in the molecule. While selecting the center atom, always put the least. Web chemistry chemistry questions and answers draw the lewis structure of ammonia (nh3). Drawing the lewis structure for nh3 it is helpful if you: Web the lewis.
Lewis Structure of NH3 YouTube
Draw the lewis structure of ammonia (nh3). + this problem has been solved! It is six for one ammonia (nh3) molecule according to the octet rule. This chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia. Draw the lewis structure for nh3. Start by counting the total number.
Draw A Lewis Structure For Nh3 If the species is an ion, add or subtract electrons corresponding to the charge of the ion. This chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia. Be sure to include any lone pairs.b) draw a vsepr diagram for ammonia. Drawing the lewis structure for nh3 it is helpful if you: Web chemistry chemistry questions and answers draw the lewis structure of ammonia (nh3).
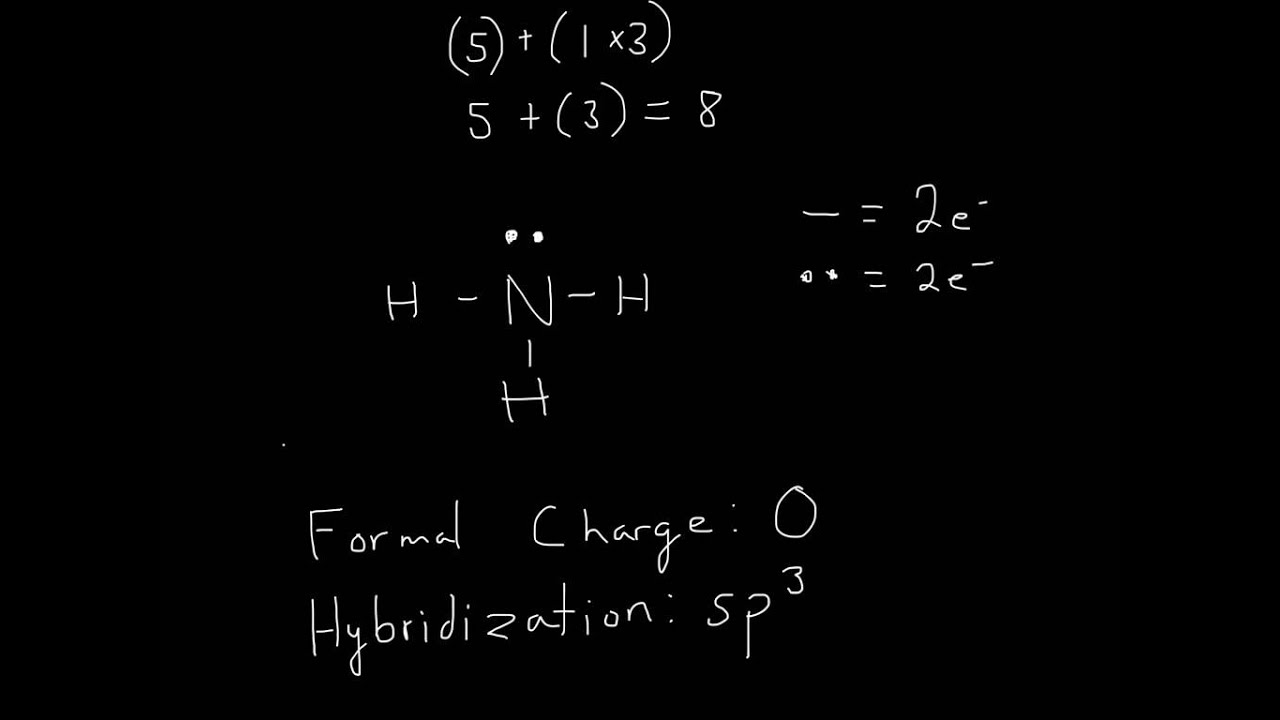
Find The Total Valence Electrons In Nh3 Molecule In Order To Find The Total Valence Electrons In Nh3 Molecule , First Of All You Should Know The Valence Electrons Present In Nitrogen Atom As.
Here, the given molecule is nh3 (ammonia). Web = 8 valence ammonia or nh3 has a total of 8 valence electrons. Select the center atom (h is always outside). A) draw the lewis structure for ammonia (nh3).
Please Include All Nonbonding Electrons.
It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. Be sure to have the correct number of electrons. Web there is really only one way to draw the lewis structure for ammonia (nh3). Web drawing lewis structures for molecules with one central atom:
Web The Lewis Structure Of Ammonia, N H 3, Would Be Three Hydrogen Atoms Bonded To A Nitrogen Atom In The Middle, With A Lone Pair Of Electrons On Top Of The Atom.
Valence electrons are the electrons in the outermost energy level of an atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. + show transcribed image text there are 3 steps to solve this one. Web to draw the lewis structure of nh3, follow these steps:
Web Follow These Simple Steps To Draw Lewis Dot Structures:
For the nh3 structure use the periodic table to find the total number of valence electrons for the nh3. Find more chemistry widgets in wolfram|alpha. This chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia. Count the total number of valence electrons to begin drawing the nh3 lewis structure, start by counting the total.