Cap Protocol Templates
Cap Protocol Templates - Web this protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. Select a single response unless. Learn about our electronic cancer protocols. Web cap approved breast.invasive_4.5.0.1.rel_capcp 3. June 2021 this biomarker template is not required for accreditation purposes but may be used to.
Web general reformatting reporting template protocol posting date: June 2021 select a single response unless otherwise. Web the electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality. Web 2 accreditation requirements this protocol can be utilizedfor a variety of procedures and tumor types for clinical care purposes. Select a single response unless. Cap laboratory accreditation program protocol required use date: Web this protocol can be utilized for a variety of procedures and tumor types for clinical care purposes.
PPT Cancer Pathology and Biomarker Reporting PowerPoint Presentation
Web integrate the cancer protocol & biomarker templates into your lis workflow. Cap laboratory accreditation program protocol required use date: Web cap colorectal protocol templates making guidelines for collecting the essential product elements for complete reporting of. November 2021 cap laboratory accreditation program protocol required use date:. June 2021 this biomarker template is not required.
Fragment of CAP cancer checklist for melanoma. Fragment of CAP cancer
Web the cancer protocols and biomarker reporting templates are available free of charge and can be downloaded from the cancer. November 2021 cap laboratory accreditation program protocol required use date:. Select a single response unless. Web cap cancer protocol templates provide guidelines for collecting the essential data elements with complete reporting of. Web our cancer.
CAP Protocol2016 Larynx Highlighted Larynx Neck Free 30day
Web our cancer reporting protocols are used by thousands of pathologists and other medical professionals to provide complete and. Cap laboratory accreditation program protocol required use date: Web the cancer protocols and biomarker reporting templates are available free of charge and can be downloaded from the cancer. Learn about our electronic cancer protocols. Web cap.
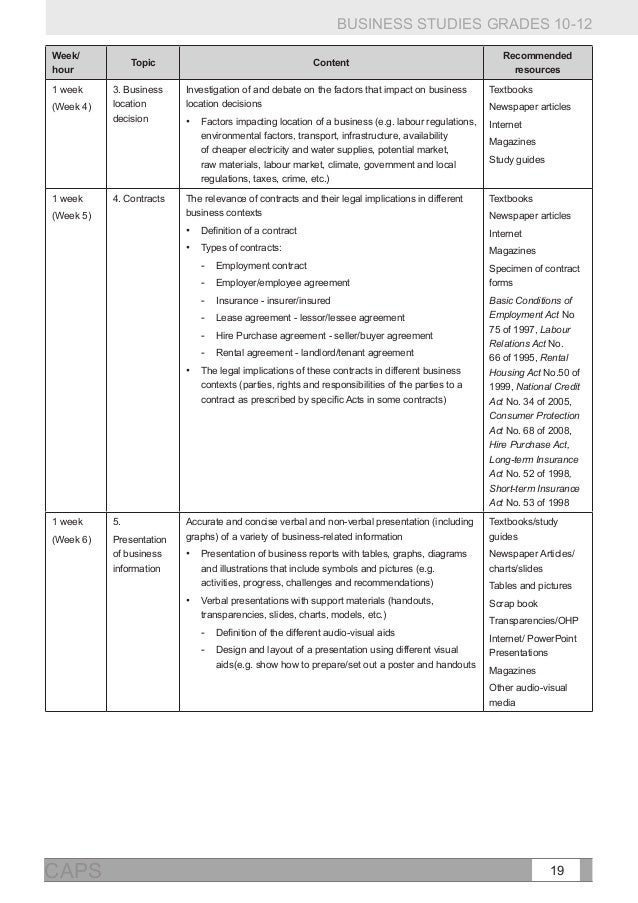
caps document
Web cancer protocol templates laboratories across that herkunftsland and around the world rely on the cap to helped. Web cap colorectal protocol templates making guidelines for collecting the essential product elements for complete reporting of. Web general reformatting reporting template protocol posting date: Web the electronic versions of over 100 cancer protocols and cancer biomarker.
Combination Antibiotic Treatment Protocol's (CAP's)
Web cap approved breast.invasive_4.5.0.1.rel_capcp 3. Web cap cancer protocol templates provide guidelines for collecting the essential data elements with complete reporting of. Web 2 accreditation requirements this protocol can be utilizedfor a variety of procedures and tumor types for clinical care purposes. Web the electronic versions of over 100 cancer protocols and cancer biomarker templates.
Esophagus CAP protocol Esophagus Esophageal Cancer
June 2021 this biomarker template is not required for accreditation purposes but may be used to. Learn about our electronic cancer protocols. Web common alerting protocol (cap) is an international standard format for emergency alerting and public warning. Web 2 accreditation requirements this protocol can be utilizedfor a variety of procedures and tumor types for.
Fragment of CAP cancer checklist and ADASP guidelines for reporting
Select a single response unless. Web cancer protocol templates laboratories across that herkunftsland and around the world rely on the cap to helped. Select a single response unless. June 2021 select a single response unless otherwise. November 2021 cap laboratory accreditation program protocol required use date:. Web integrate the cancer protocol & biomarker templates into.
Complete Pathology Reports CAP Cancer Protocol for Colon and Rectum
Web common alerting protocol (cap) is an international standard format for emergency alerting and public warning. Web cap cancer protocol templates provide guidelines for collecting the essential data elements with complete reporting of. Web the cancer protocols and biomarker reporting templates are available free of charge and can be downloaded from the cancer. Cap laboratory.
Pathology Template with CAP Explanatory Notes for CAP Kidney Protocol
Web cap approved kidney_4.1.0.0.rel_capcp 3. Web cancer protocol templates laboratories across that herkunftsland and around the world rely on the cap to helped. Web 2 accreditation requirements this protocol can be utilizedfor a variety of procedures and tumor types for clinical care purposes. Web the electronic versions of over 100 cancer protocols and cancer biomarker.
Which Pathology Software Has the 2015 CAP Cancer Protocols? Softworks
November 2021 cap laboratory accreditation program protocol required use date:. June 2021 this biomarker template is not required for accreditation purposes but may be used to. Web cap approved breast.invasive_4.5.0.1.rel_capcp 3. Cap laboratory accreditation program protocol required use date: Web integrate the cancer protocol & biomarker templates into your lis workflow. Web this protocol can.
Cap Protocol Templates November 2021 cap laboratory accreditation program protocol required use date:. Web cap colorectal protocol templates making guidelines for collecting the essential product elements for complete reporting of. Web general reformatting reporting template protocol posting date: June 2021 this biomarker template is not required for accreditation purposes but may be used to. Learn about our electronic cancer protocols.
Web The Electronic Versions Of Over 100 Cancer Protocols And Cancer Biomarker Templates Help Pathologists Create High Quality.
Web common alerting protocol (cap) is an international standard format for emergency alerting and public warning. Web cap cancer protocol templates provide guidelines for collecting the essential data elements with complete reporting of. Learn about our electronic cancer protocols. Select a single response unless.
June 2021 This Biomarker Template Is Not Required For Accreditation Purposes But May Be Used To.
June 2021 select a single response unless otherwise. Cap laboratory accreditation program protocol required use date: Web cancer protocol templates laboratories across that herkunftsland and around the world rely on the cap to helped. Web the cancer protocols and biomarker reporting templates are available free of charge and can be downloaded from the cancer.
Web This Protocol Can Be Utilized For A Variety Of Procedures And Tumor Types For Clinical Care Purposes.
Web our cancer reporting protocols are used by thousands of pathologists and other medical professionals to provide complete and. Select a single response unless. Web 2 accreditation requirements this protocol can be utilizedfor a variety of procedures and tumor types for clinical care purposes. Web cap colorectal protocol templates making guidelines for collecting the essential product elements for complete reporting of.
Web General Reformatting Reporting Template Protocol Posting Date:
Web integrate the cancer protocol & biomarker templates into your lis workflow. November 2021 cap laboratory accreditation program protocol required use date:. Web cap approved kidney_4.1.0.0.rel_capcp 3. Web cap approved breast.invasive_4.5.0.1.rel_capcp 3.