Activation Energy Drawing
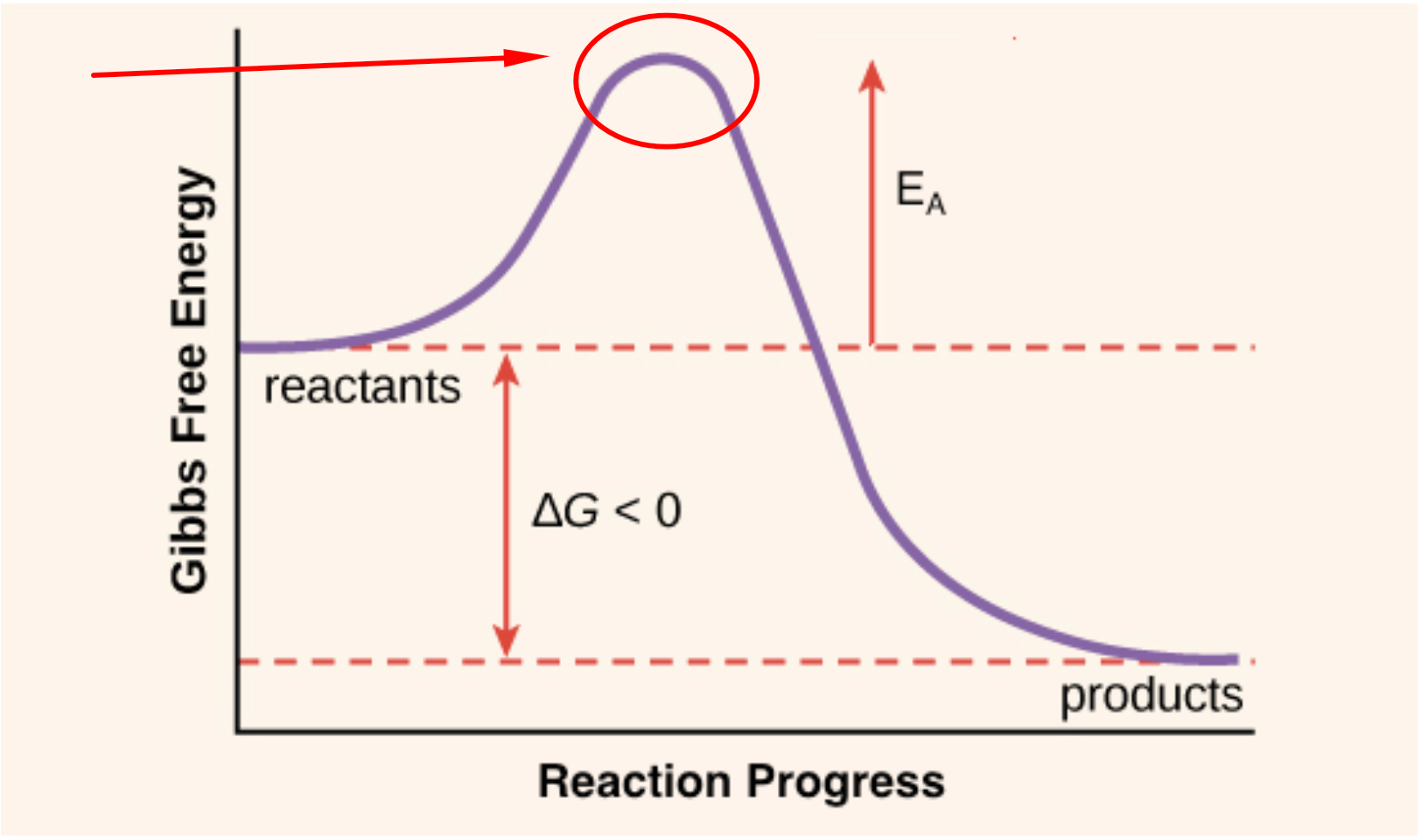
Activation Energy Drawing - The formula used to find the value of activation energy, e a is; Web the potential energy diagrams for a reaction with (a) δe < 0 and (b) δe > 0 illustrate the change in the potential energy of the system as reactants are converted to products. In both cases, ea e a is positive. Web the activation energy for a reaction is the minimum energy that colliding particles must have in order to undergo a reaction. E a = activation energy.
At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in. Web the activation energy is what determines the kinetics of a reaction: Web activation energy is indicated by the symbol e a and has units of joules (j), kilojoules per mole (kj/mol), or kilocalories per mole (kcal/mol). Web the activation energy is what determines the kinetics of a reaction: Web a physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. Label the vertical axis potential energy and the horizontal axis reaction coordinate. In both cases, ea e a is positive.
Activation Energy The Secret to Getting Started and Getting Finished
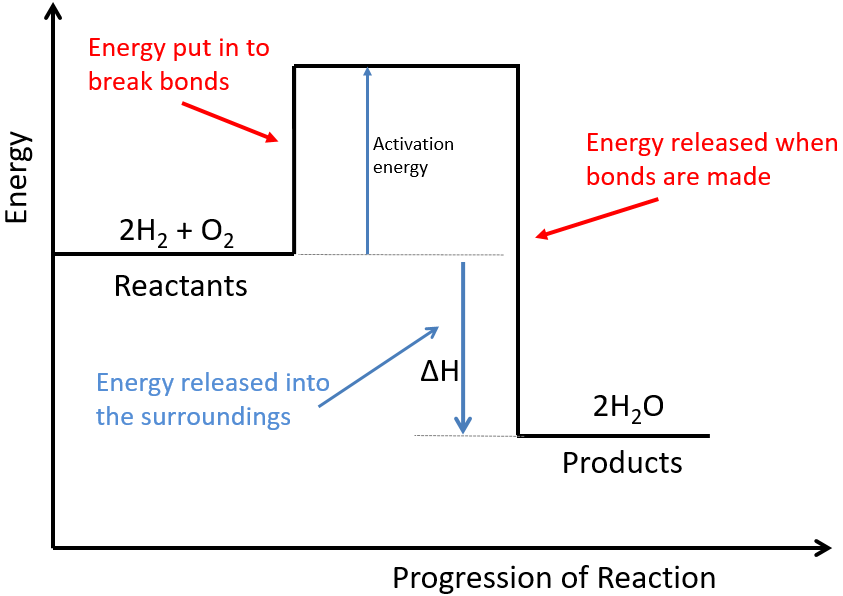
Web the activation energy is what determines the kinetics of a reaction: Web activation energy is indicated by the symbol e a and has units of joules (j), kilojoules per mole (kj/mol), or kilocalories per mole (kcal/mol). Even exothermic reactions, such as burning a candle, require energy input. If the initial state has a lower.
Energy Diagram — Overview & Parts Expii
Web 6/02 base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Web the activation energy (\(e_a\)), labeled \(\delta{g^{\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. At the very top of.
Reaction Coordinate Diagrams College Chemistry
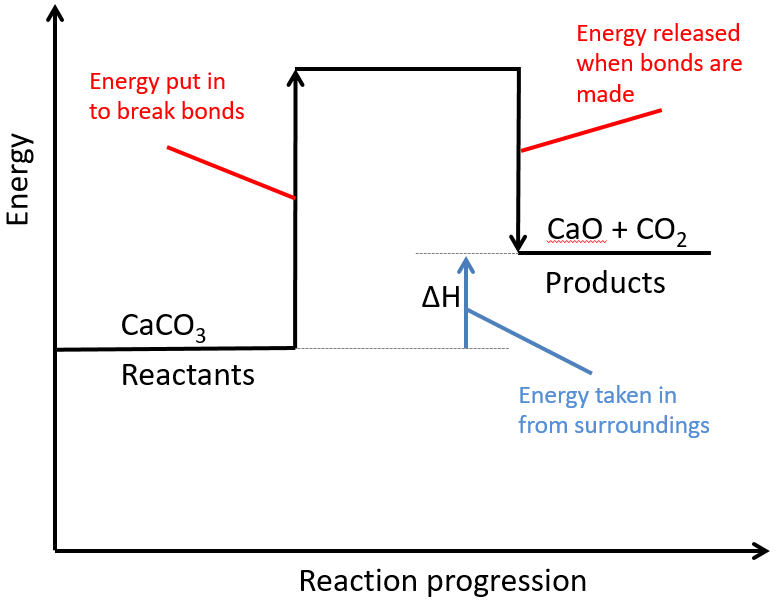
State one reason, in terms of energy, to support your answer. Web in a diagram, activation energy is graphed as the height of an energy barrier between two minimum points of potential energy. Web drawing reaction profiles reaction profiles show relative energies. The higher the energy hill, the slower the reaction. The energy profile can.
Activation energy vector illustration example diagram Physics and
At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in. The activation energy (e a) of a reaction is measured in kilojoules per mole (kj/mol) or kilocalories per mole (kcal/mol). Label the vertical axis potential energy and the horizontal.
Activation Energy The Secret to Getting Started and Getting Finished
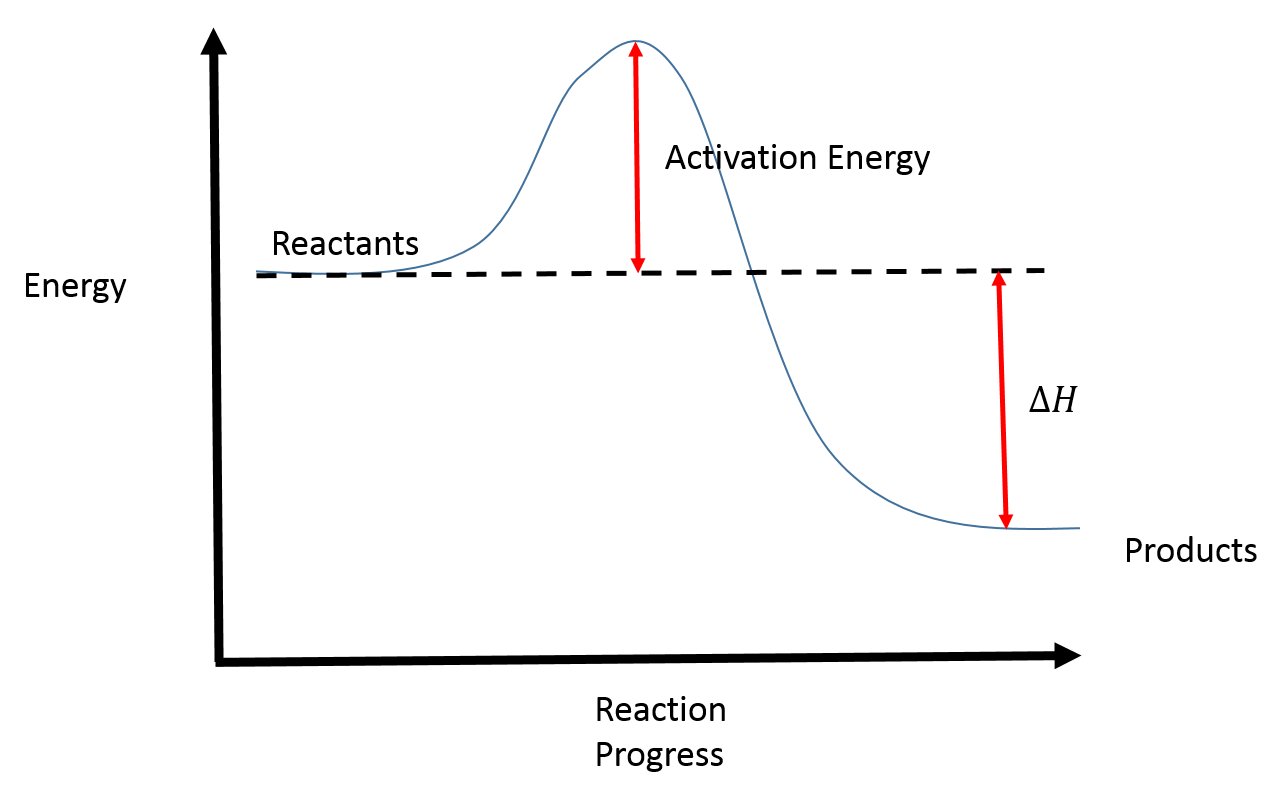
Web the energy difference between reactants and the transition state is called the activation energy, δg‡, and determines how rapidly the reaction occurs at a given temperature. Web a physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy.
314 (Triple only) draw and explain reaction profile diagrams showing
The higher the energy hill, the slower the reaction. Draw the energy level diagram. Web the energy difference between reactants and the transition state is called the activation energy, δg‡, and determines how rapidly the reaction occurs at a given temperature. Web the activation energy is what determines the kinetics of a reaction: Web 6/02.
Activation Energy Definition, Formula, SI Units, Examples, Calculation
Web solution we can obtain the activation energy by plotting ln k versus , knowing that the slope will be equal to. For a reaction such as the one shown in (b), e a must be greater than δe. R = gas constant = 8.34j/k/mol =8.314/1000 kj/k/mol = 2 cal/k/mol = 0.0821 lit atm/k/mol. Web.
What are activation energies? Socratic
Web the activation energy is what determines the kinetics of a reaction: At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in the process of breaking and forming. The higher the energy hill, the slower the reaction. (jerry crimson.
314 (Triple only) draw and explain reaction profile diagrams showing
Web the potential energy diagrams for a reaction with (a) δe < 0 and (b) δe > 0 illustrate the change in the potential energy of the system as reactants are converted to products. Draw and label two short horizontal lines to mark the energies of the reactants and products. At the very top of.
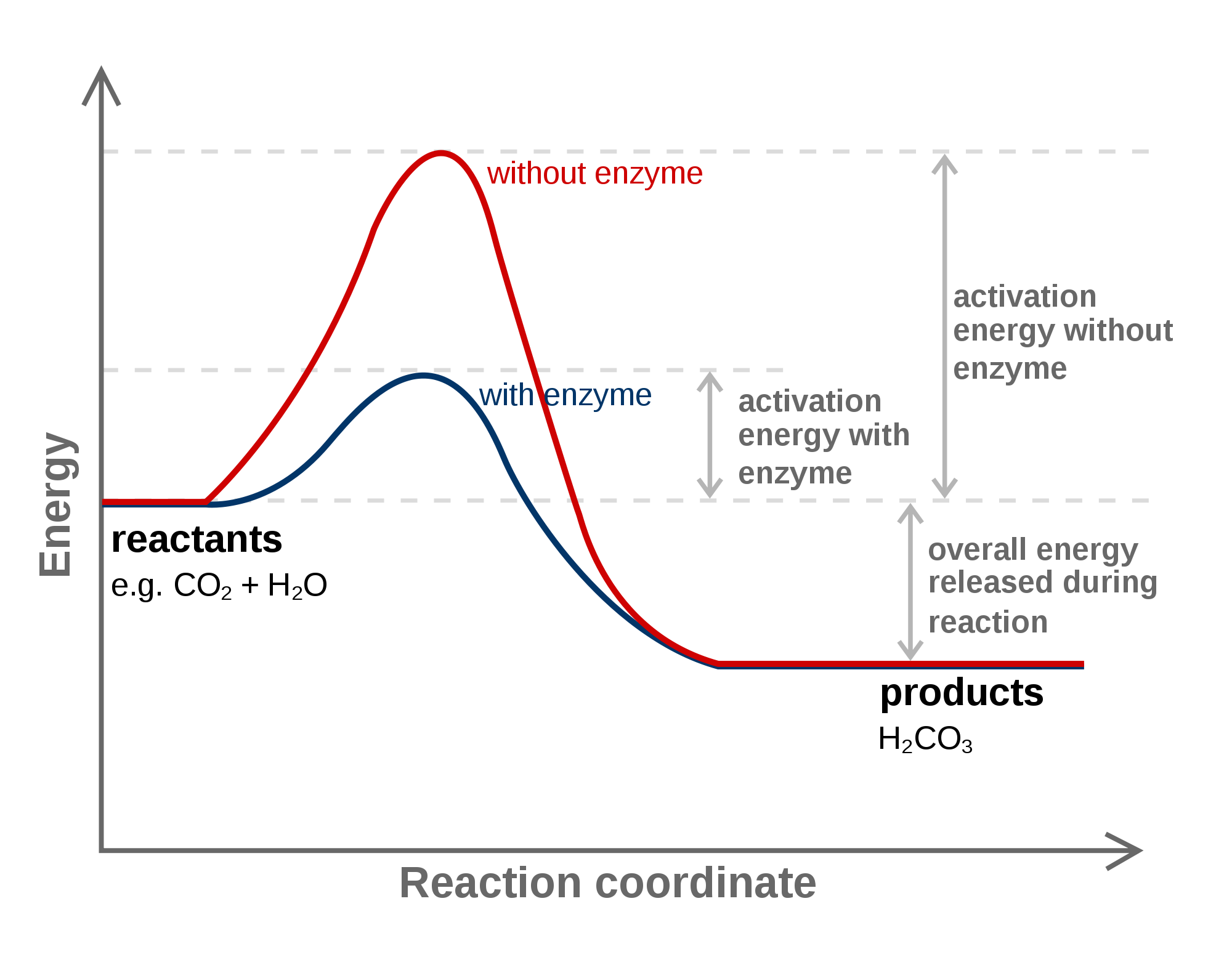
Enzymes Lower The Activation Energy Of A Reaction btccasting
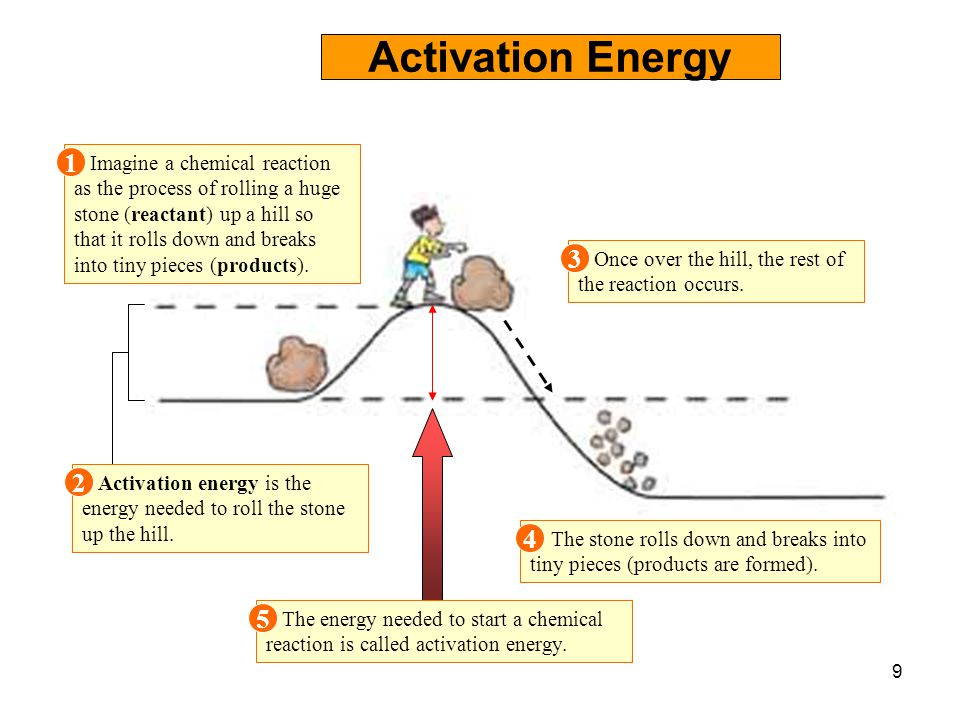
Once the reaction has obtained this amount of energy, it must continue on. Web exothermic energy diagram: Web the activation energy for a reaction is the minimum energy that colliding particles must have in order to undergo a reaction. Label the vertical axis potential energy and the horizontal axis reaction coordinate. Web the activation energy.
Activation Energy Drawing Web in the arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (e a) of a reaction is measured in kilojoules per mole (kj/mol) or kilocalories per mole (kcal/mol). The higher the energy hill, the slower the reaction. At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in the process of breaking and forming. In both cases, ea e a is positive.
Web The Activation Energy Is What Determines The Kinetics Of A Reaction:
When drawing a reaction profile, we should be able to label the relative energies of the reactants as compared to the products. If the initial state has a lower potential energy than the. Once the reaction has obtained this amount of energy, it must continue on. Taking log on both sides.
Web Drawing Reaction Profiles Reaction Profiles Show Relative Energies.
Draw and label two short horizontal lines to mark the energies of the reactants and products. Web 6/02 base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. The activated complex is an unstable, intermediate product that is formed during the reaction. At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in.
Some Reactions Occur Readily At Room Temperature Because The Reacting Particles Already Have The Requisite Activation Energy At That Temperature.
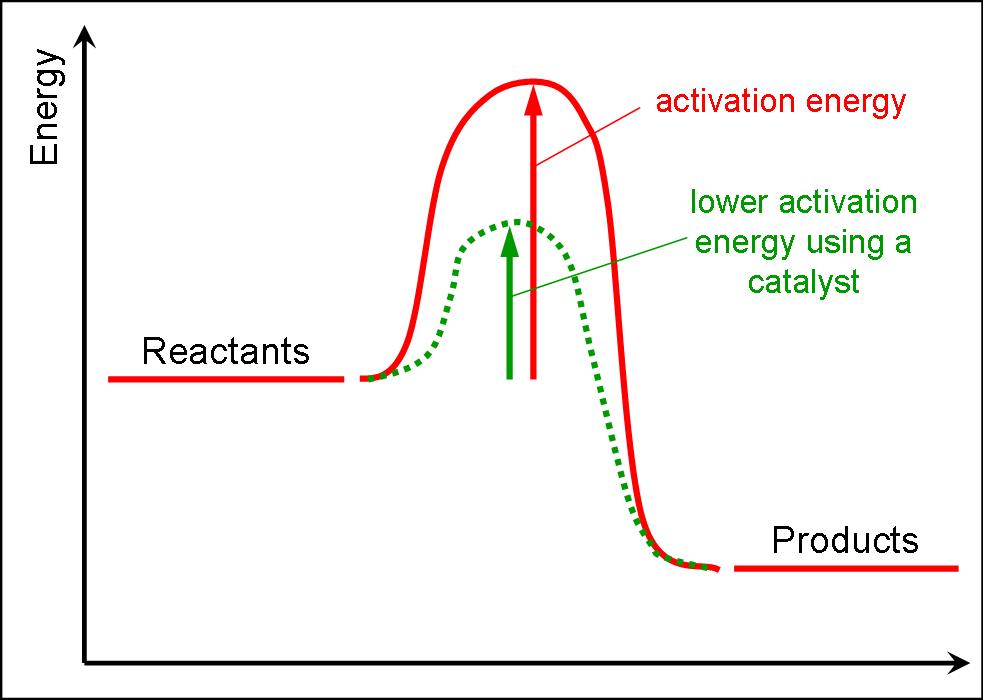
Even exothermic reactions, such as burning a candle, require energy input. An enzyme or catalyst lowers the activation energy. In this video, i go over how to properly label and explain a reaction mechanism diagram which. At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in.
The Higher The Energy Hill, The Slower The Reaction.
E a = activation energy. The minimum points are the energies of the stable reactants and products. In both cases, ea e a is positive. Web the activation energy is what determines the kinetics of a reaction: